Preparedness for the Dengue Epidemic: Vaccine as a Viable Approach
- PMID: 36423035
- PMCID: PMC9697487
- DOI: 10.3390/vaccines10111940
Preparedness for the Dengue Epidemic: Vaccine as a Viable Approach
Abstract
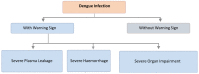
Dengue fever is one of the significant fatal mosquito-borne viral diseases and is considered to be a worldwide problem. Aedes mosquito is responsible for transmitting various serotypes of dengue viruses to humans. Dengue incidence has developed prominently throughout the world in the last ten years. The exact number of dengue cases is underestimated, whereas plenty of cases are misdiagnosed as alternative febrile sicknesses. There is an estimation that about 390 million dengue cases occur annually. Dengue fever encompasses a wide range of clinical presentations, usually with undefinable clinical progression and outcome. The diagnosis of dengue depends on serology tests, molecular diagnostic methods, and antigen detection tests. The therapeutic approach relies completely on supplemental drugs, which is far from the real approach. Vaccines for dengue disease are in various stages of development. The commercial formulation Dengvaxia (CYD-TDV) is accessible and developed by Sanofi Pasteur. The vaccine candidate Dengvaxia was inefficient in liberating a stabilized immune reaction toward different serotypes (1-4) of dengue fever. Numerous promising vaccine candidates are now being developed in preclinical and clinical stages even though different serotypes of DENV exist that worsen the situation for a vaccine to be equally effective for all serotypes. Thus, the development of an efficient dengue fever vaccine candidate requires time. Effective dengue fever management can be a multidisciplinary challenge, involving international cooperation from diverse perspectives and expertise to resolve this global concern.
Keywords: Aedes mosquito; clinical presentations; dengue; dengue vaccines; diagnosis.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





References
-
- Afreen N., Deeba F., Khan W.H., Haider S.H., Kazim S.N., Ishrat R., Naqvi I.H., Shareef M.Y., Broor S., Ahmed A., et al. Molecular characterization of dengue and chikungunya virus strains circulating in New Delhi, India. Microbiol. Immunol. 2014;58:688–696. doi: 10.1111/1348-0421.12209. - DOI - PubMed
-
- Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., Dandona L. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. - PMC - PubMed
-
- Khan W.H., Ahmad R., Khan N., Ansari M.A. SARS-CoV-2 Variants Associated Challenges in the Ongoing Vaccination of COVID-19. In: Raad H., editor. Prime Archives in Virology. Volume 1. Prime Archives in Virology; Hyderabad, India: 2022. pp. 1–59.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

