The future of engineered immune cell therapies
- PMID: 36423279
- PMCID: PMC9919886
- DOI: 10.1126/science.abq6990
The future of engineered immune cell therapies
Abstract
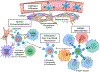
Immune cells are being engineered to recognize and respond to disease states, acting as a "living drug" when transferred into patients. Therapies based on engineered immune cells are now a clinical reality, with multiple engineered T cell therapies approved for treatment of hematologic malignancies. Ongoing preclinical and clinical studies are testing diverse strategies to modify the fate and function of immune cells for applications in cancer, infectious disease, and beyond. Here, we discuss current progress in treating human disease with immune cell therapeutics, emerging strategies for immune cell engineering, and challenges facing the field, with a particular emphasis on the treatment of cancer, where the most effort has been applied to date.
Figures
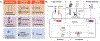

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

