The impact of benzodiazepine exposure on treatment retention in an open-access methadone program: A retrospective cohort study
- PMID: 36423462
- PMCID: PMC9777057
- DOI: 10.1016/j.drugalcdep.2022.109707
The impact of benzodiazepine exposure on treatment retention in an open-access methadone program: A retrospective cohort study
Abstract
Background: Open-access opioid treatment programs (OTP) offer same-day access to methadone without an appointment and aim to minimize treatment barriers that often reduce admission and/or retention. We explored whether patients with benzodiazepine exposure at treatment entry would have similar 12-month retention compared to those without benzodiazepine exposure.
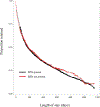
Methods: We conducted a retrospective cohort study of 2968 patients consecutively initiated on methadone between January 2015 and February 2017 at an open-access OTP. The sample was stratified into benzodiazepine-exposed and nonexposed groups based on intake urine toxicology. Group comparison of 12-month retention was conducted. Kaplan Meier analysis compared time to methadone treatment discontinuation between groups with a log-rank test. Multivariable Cox regression was performed to compare retention by baseline benzodiazepine exposure with adjustment for confounders.
Results: Overall, 31% of patients with benzodiazepine exposure (n = 171) and 31% without exposure (n = 2423) were retained at 12 months (p = 0.95). Median treatment duration was 182 days (95% CI, 152-239) and 175 days (95% CI, 156-196) for patients with and without benzodiazepine exposure, respectively. Kaplan-Meier analysis showed no significant difference in treatment duration between groups (log-rank test p = 0.73). Cox regression found no difference in treatment retention between groups (adjusted Hazard Ratio= 1.03, 95% CI, 0.91-1.16).
Conclusions: In this cohort of patients receiving methadone at an open-access OTP, benzodiazepine exposure at intake was not observed to impact 12-month treatment retention or duration. These findings support U.S. Food and Drug Administration (FDA) recommendations to not withhold medications for opioid use disorder from patients taking benzodiazepines.
Keywords: Benzodiazepine; Methadone; Opioid use disorder; Treatment retention.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest All authors have no declarations or conflicts of interest to report.
Figures
Similar articles
-
The impact of benzodiazepine use in patients enrolled in opioid agonist therapy in Northern and rural Ontario.Harm Reduct J. 2017 Jan 26;14(1):6. doi: 10.1186/s12954-017-0134-5. Harm Reduct J. 2017. PMID: 28122579 Free PMC article.
-
Evaluating the Impact of Prescribed Versus Nonprescribed Benzodiazepine Use in Methadone Maintenance Therapy: Results From a Population-based Retrospective Cohort Study.J Addict Med. 2019 May/Jun;13(3):182-187. doi: 10.1097/ADM.0000000000000476. J Addict Med. 2019. PMID: 30543543 Free PMC article.
-
Bridge clinic implementation of "72-hour rule" methadone for opioid withdrawal management: Impact on opioid treatment program linkage and retention in care.Drug Alcohol Depend. 2022 Jul 1;236:109497. doi: 10.1016/j.drugalcdep.2022.109497. Epub 2022 May 14. Drug Alcohol Depend. 2022. PMID: 35607834
-
Three-year retention in methadone opioid agonist treatment: A survival analysis of clients by dose, area deprivation, and availability of alcohol and cannabis outlets.Drug Alcohol Depend. 2018 Dec 1;193:63-68. doi: 10.1016/j.drugalcdep.2018.08.024. Epub 2018 Oct 6. Drug Alcohol Depend. 2018. PMID: 30340146
-
Opioid treatment program and community pharmacy collaboration for methadone maintenance treatment: results from a feasibility clinical trial.Addiction. 2022 Feb;117(2):444-456. doi: 10.1111/add.15641. Epub 2021 Aug 16. Addiction. 2022. PMID: 34286886 Free PMC article. Clinical Trial.
Cited by
-
Barriers to opioid use disorder treatment among people who use drugs in the rural United States: A qualitative, multi-site study.Soc Sci Med. 2024 Apr;346:116660. doi: 10.1016/j.socscimed.2024.116660. Epub 2024 Feb 13. Soc Sci Med. 2024. PMID: 38484417 Free PMC article.
-
A qualitative study of benzodiazepine/z-drug and opioid co-use patterns and overdose risk.Harm Reduct J. 2025 Feb 27;22(1):24. doi: 10.1186/s12954-025-01153-8. Harm Reduct J. 2025. PMID: 40016748 Free PMC article.
References
-
- Eibl JK, Wilton AS, Franklyn AM, Kurdyak P, & Marsh DC (2019, May/Jun). Evaluating the Impact of Prescribed Versus Nonprescribed Benzodiazepine Use in Methadone Maintenance Therapy: Results From a Population-based Retrospective Cohort Study. J Addict Med, 13(3), 182–187. 10.1097/adm.0000000000000476 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical