Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases
- PMID: 36424489
- PMCID: PMC10257351
- DOI: 10.1038/s41587-022-01527-4
Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases
Abstract
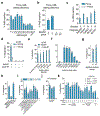
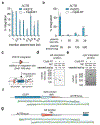
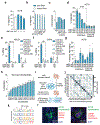
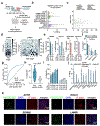
Programmable genome integration of large, diverse DNA cargo without DNA repair of exposed DNA double-strand breaks remains an unsolved challenge in genome editing. We present programmable addition via site-specific targeting elements (PASTE), which uses a CRISPR-Cas9 nickase fused to both a reverse transcriptase and serine integrase for targeted genomic recruitment and integration of desired payloads. We demonstrate integration of sequences as large as ~36 kilobases at multiple genomic loci across three human cell lines, primary T cells and non-dividing primary human hepatocytes. To augment PASTE, we discovered 25,614 serine integrases and cognate attachment sites from metagenomes and engineered orthologs with higher activity and shorter recognition sequences for efficient programmable integration. PASTE has editing efficiencies similar to or exceeding those of homology-directed repair and non-homologous end joining-based methods, with activity in non-dividing cells and in vivo with fewer detectable off-target events. PASTE expands the capabilities of genome editing by allowing large, multiplexed gene insertion without reliance on DNA repair pathways.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures












Comment in
-
Novel recombinases for large DNA insertions.Nat Biotechnol. 2023 Apr;41(4):471-472. doi: 10.1038/s41587-022-01600-y. Nat Biotechnol. 2023. PMID: 36424492 No abstract available.
-
Pasting in large DNA sequences.Nat Rev Drug Discov. 2023 Feb;22(2):99. doi: 10.1038/d41573-023-00011-1. Nat Rev Drug Discov. 2023. PMID: 36631698 No abstract available.
-
PASTE: The Way Forward for Large DNA Insertions.CRISPR J. 2023 Feb;6(1):2-4. doi: 10.1089/crispr.2023.0001. Epub 2023 Jan 30. CRISPR J. 2023. PMID: 36716261 No abstract available.
References
-
- Anzalone AV, Koblan LW & Liu DR Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol 38, 824–844 (2020). - PubMed
-
- Wright AV, Nuñez JK & Doudna JA Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell 164, 29–44 (2016). - PubMed
-
- Nami F et al. Strategies for In Vivo Genome Editing in Nondividing Cells. Trends Biotechnol. 36, 770–786 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

