Diversity of endophytic fungi isolated from different plant parts of Acacia mangium, and antagonistic activity against Ceratocystis fimbriata, a causal agent of Ceratocystis wilt disease of A. mangium in Malaysia
- PMID: 36425026
- PMCID: PMC9679781
- DOI: 10.3389/fmicb.2022.887880
Diversity of endophytic fungi isolated from different plant parts of Acacia mangium, and antagonistic activity against Ceratocystis fimbriata, a causal agent of Ceratocystis wilt disease of A. mangium in Malaysia
Abstract
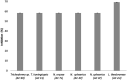
Acacia mangium is an important wood for commercial products especially pulp and medium-density fibreboard. However, it is susceptible to Ceratocystis fimbriata infection, leading to Ceratocystis wilt. Therefore, the present work aimed to (i) establish the diversity of endophytic fungi in different plant parts of A. mangium,and (ii) evaluate the antifungal potentials of the isolated and identified endophytic fungi against C. fimbriata. Endophytic fungal identification was conducted by PCR amplification and sequencing of the internal transcribed spacer 1 (ITS1) and ITS4 regions of nuclear ribosomal DNA. A total of 66 endophytic fungi were successfully isolated from different parts of A. mangium; leaf (21), stem (13), petiole (12), root (9), flower (6), and fruit (5). The endophytic fungal isolates belonged to Ascomycota (95.5%) and Zygomycota (4.5%). For Ascomycota 13 genera were identified: Trichoderma (28.6%), Nigrospora (28.6%), Pestalotiopsis (12.7%), Lasiodiplodia (9.5%), Aspergillus (6.3%), Sordariomycetes (3%), and Neopestalotiopsis, Pseudopestalotiopsis, Eutiarosporella, Curvularia, Fusarium, Penicillium, and Hypoxylon each with a single isolate. For Zygomycota, only Blakeslea sp. (5%) was isolated. Against C. fimbriata, Trichoderma koningiopsis (AC 1S) from stem, Nigrospora oryzae (AC 7L) from leaf, Nigrospora sphaerica (AC 3F) from the flower, Lasiodiplodia sp. (AC 2 U) from fruit, Nigrospora sphaerica (AC 4P) from petiole, and Trichoderma sp. (AC 9R) from root exhibited strong inhibition for C. fimbriata between 58.33 to 69.23%. Thus, it can be concluded that certain endophytic fungi of A. mangium have the potential to be harnessed as anti-Ceratocystis agent in future biotechnological applications.
Keywords: Acacia mangium; Ceratocystis fimbriata; Ceratocystis wilt; antagonism; endophytic fungi.
Copyright © 2022 Ahmad, Zahari, Mohtar, Wan-Muhammad-Azrul, Hishamuddin, Samsudin, Hassan and Terhem.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anitha A., Rabeeth M. (2010). Degradation of fungal cell walls of phytopathogenic fungi by lytic enzyme of Streptomyces griseus. African J. Plant Sci. 4, 61–66.
-
- Arnold A. E., Maynard Z., Gilbert G. S. (2001). Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity. Mycol. Res. 105, 1502–1507. doi: 10.1017/S0953756201004956 - DOI
-
- Blaedow R. A. (2009). Use of the systemic fungicide propiconazole for oak wilt management: an assessment of uncharacterized host-pathogen-fungicide interactions. University of Minnesota.
-
- Brawner J., Japarudin Y., Lapammu M., Rauf R., Boden D., Wingfield M. J. (2015). Evaluating the inheritance of Ceratocystis acaciivora symptom expression in a diverse Acacia mangium breeding population. South. For. 77, 83–90. doi: 10.2989/20702620.2015.1007412 - DOI
LinkOut - more resources
Full Text Sources

