Detection of Antinuclear Antibodies Targeting Intracellular Signal Transduction, Metabolism, Apoptotic Processes and Cell Death in Critical COVID-19 Patients
- PMID: 36425144
- PMCID: PMC9652015
- DOI: 10.4084/MJHID.2022.076
Detection of Antinuclear Antibodies Targeting Intracellular Signal Transduction, Metabolism, Apoptotic Processes and Cell Death in Critical COVID-19 Patients
Abstract
Background and objectives: The heterogeneity of the coronavirus disease of 2019 (COVID-19) lies within its diverse symptoms and severity, ranging from mild to lethal. Acute respiratory distress syndrome (ARDS) is a leading cause of mortality in COVID-19 patients, characterized by a hyper cytokine storm. Autoimmunity is proposed to occur as a result of COVID-19, given the high similarity of the immune responses observed in COVID-19 and autoimmune diseases. Here, we investigate the level of autoimmune antibodies in COVID-19 patients with different severities.
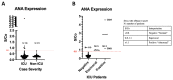
Results: Initial screening for antinuclear antibodies (ANA) IgG using ELISA revealed that 1.58% (2/126) and 4% (5/126) of intensive care unit (ICU) COVID-19 cases expressed strong and moderate ANA levels, respectively. An additional sample was positive with immunofluorescence assays (IFA) screening. However, all the non-ICU cases (n=273) were ANA negative using both assays. Samples positive for ANA were further confirmed with large-scale autoantibody screening by phage immunoprecipitation-sequencing (PhIP-Seq). The majority of the ANA-positive samples showed "speckled" ANA pattern by microscopy and revealed autoantibody specificities that targeted proteins involved in intracellular signal transduction, metabolism, apoptotic processes, and cell death by PhIP-Seq; further denoting reactivity to nuclear and cytoplasmic antigens.
Conclusion: Our results further support the notion of routine screening for autoimmune responses in COVID-19 patients, which might help improve disease prognosis and patient management. Further, results provide compelling evidence that ANA-positive individuals should be excluded from being donors for convalescent plasma therapy in the context of COVID-19.
Keywords: ANA; Autoimmunity; COVID-19; Coronavirus; ICU.
Conflict of interest statement
Competing interests: The authors declare no conflict of Interest.
Figures


Similar articles
-
Automated antinuclear immunofluorescence antibody screening: a comparative study of six computer-aided diagnostic systems.Autoimmun Rev. 2014 Mar;13(3):292-8. doi: 10.1016/j.autrev.2013.10.015. Epub 2013 Nov 9. Autoimmun Rev. 2014. PMID: 24220268 Review.
-
Mild-to-moderate COVID-19 does not predispose to the development of autoimmune rheumatic diseases or autoimmune-based thrombosis.Scand J Immunol. 2023 Nov;98(5):e13313. doi: 10.1111/sji.13313. Epub 2023 Jul 26. Scand J Immunol. 2023. PMID: 38441212
-
[Investigation of antinuclear antibodies in chronic hepatitis B patients].Mikrobiyol Bul. 2018 Oct;52(4):425-430. doi: 10.5578/mb.67262. Mikrobiyol Bul. 2018. PMID: 30522427 Turkish.
-
Antinuclear antibodies (ANA) screening by enzyme immunoassay with nuclear HEp-2 cell extract and recombinant antigens: analytical and clinical evaluation.Clin Biochem. 2002 Sep;35(6):463-9. doi: 10.1016/s0009-9120(02)00342-9. Clin Biochem. 2002. PMID: 12413607
-
Autoantibodies Against DFS70/LEDGF Exclusion Markers for Systemic Autoimmune Rheumatic Diseases (SARD).Clin Lab. 2016;62(4):499-517. doi: 10.7754/clin.lab.2015.150905. Clin Lab. 2016. PMID: 27215068 Review.
Cited by
-
Brain-targeting autoantibodies in patients with dementia.Front Neurol. 2024 Jul 10;15:1412813. doi: 10.3389/fneur.2024.1412813. eCollection 2024. Front Neurol. 2024. PMID: 39050125 Free PMC article.
-
Effect of serum autoantibodies on the COVID-19 patient's prognosis.Front Microbiol. 2023 Nov 30;14:1259960. doi: 10.3389/fmicb.2023.1259960. eCollection 2023. Front Microbiol. 2023. PMID: 38107861 Free PMC article.
-
PhIP-Seq: methods, applications and challenges.Front Bioinform. 2024 Sep 4;4:1424202. doi: 10.3389/fbinf.2024.1424202. eCollection 2024. Front Bioinform. 2024. PMID: 39295784 Free PMC article. Review.
References
-
- Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, Garforth SJ, Herrera NG, Jangra RK, Morano NC, Orner E, Sy S, Chandran K, Dziura J, Almo SC, Ring A, Keller MJ, Herold KC, Herold BC. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020 Oct 7;12(564):eabd5487. doi: 10.1126/scitranslmed.abd5487. - DOI - PMC - PubMed
-
- Metzemaekers M, Cambier S, Blanter M, Vandooren J, de Carvalho AC, Malengier-Devlies B, Vanderbeke L, Jacobs C, Coenen S, Martens E, Portner N, Vanbrabant L, Van Mol P, Van Herck Y, Van Aerde N, Hermans G, Gunst J, Borin A, Toledo N, Pereira B, Dos Sp Gomes AB, Primon Muraro S, Fabiano de Souza GS, Farias A, Proenca-Modena JL, Vinolo RMA, Marques PE, Wouters C, Wauters E, Struyf S, Matthys P, Opdenakker G, Marques RE, Wauters J, Gouwy M, Proost P CONTAGIOUS Consortium. Kinetics of peripheral blood neutrophils in severe coronavirus disease. Clin Transl Immunology. 2019 2021 Apr 29;10(4):e1271. doi: 10.1002/cti2.1271. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

