Polymer-tethered glyconanoparticle colourimetric biosensors for lectin binding: structural and experimental parameters to ensure a robust output
- PMID: 36425181
- PMCID: PMC9672907
- DOI: 10.1039/d2ra06265h
Polymer-tethered glyconanoparticle colourimetric biosensors for lectin binding: structural and experimental parameters to ensure a robust output
Abstract
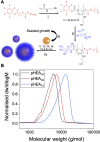
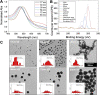
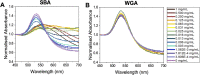
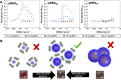
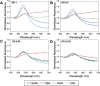
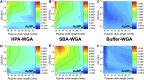
Glycan-lectin interactions play essential roles in biology; as the site of attachment for pathogens, cell-cell communication, and as crucial players in the immune system. Identifying if a new glycan (natural or unnatural) binds a protein partner, or if a new protein (or mutant) binds a glycan remains a non-trivial problem, with few accessible or low-cost tools available. Micro-arrays allow for the interrogation of 100's of glycans but are not widely available in individual laboratories. Biophysical techniques such as isothermal titration calorimetry, surface plasmon resonance spectrometry, biolayer interferometry and nuclear magnetic resonance spectroscopy all provide detailed understanding of glycan binding but are relatively expensive. Glycosylated plasmonic nanoparticles based on gold cores with polymeric tethers have emerged as biosensors to detect glycan-protein binding, based on colourimetric (red to blue) outputs which can be easily interpreted by a simple UV-visible spectrometer or by eye. Despite the large number of reports there are no standard protocols for each system or recommended start points, to allow a new user to deploy this technology. Here we explore the key parameters of nanoparticle size, polymeric tether length and gold concentration to provide some guidelines for how polymer-tethered glycosylated gold nanoparticles can be used to probe a new glycan/protein interactions, with minimal optimisation barriers. This work aimed to remove the need to explore chemical and nanoparticle space and hence remove a barrier for other users when deploying this system. We show that the concentration of the gold core is crucial to balance strong responses versus false positives and recommend a gold core size and polymer tether length which balances sufficient colloidal stability and output. Whilst subtle differences between glycans/lectins will impact the outcomes, these parameters should enable a lab user to quickly evaluate binding using minimal quantities of the glycan and lectin, to select candidates for further study.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Glycan-Gold Nanoparticles as Multifunctional Probes for Multivalent Lectin-Carbohydrate Binding: Implications for Blocking Virus Infection and Nanoparticle Assembly.J Am Chem Soc. 2020 Oct 21;142(42):18022-18034. doi: 10.1021/jacs.0c06793. Epub 2020 Sep 29. J Am Chem Soc. 2020. PMID: 32935985
-
Polyvalent Glycomimetic-Gold Nanoparticles Revealing Critical Roles of Glycan Display on Multivalent Lectin-Glycan Interaction Biophysics and Antiviral Properties.JACS Au. 2024 Aug 15;4(8):3295-3309. doi: 10.1021/jacsau.4c00610. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211605 Free PMC article.
-
Glycan heterogeneity on gold nanoparticles increases lectin discrimination capacity in label-free multiplexed bioassays.Analyst. 2016 Jul 21;141(14):4305-12. doi: 10.1039/c6an00549g. Epub 2016 May 16. Analyst. 2016. PMID: 27181289 Free PMC article.
-
Biophysical Analyses for Probing Glycan-Protein Interactions.Adv Exp Med Biol. 2018;1104:119-147. doi: 10.1007/978-981-13-2158-0_7. Adv Exp Med Biol. 2018. PMID: 30484247 Free PMC article. Review.
-
Biophysical characterization of lectin-glycan interactions for therapeutics, vaccines and targeted drug-delivery.Future Med Chem. 2014;6(18):2113-29. doi: 10.4155/fmc.14.130. Future Med Chem. 2014. PMID: 25531972 Review.
Cited by
-
Glycopolymer-Functionalized Gold Nanoparticles for the Detection of Western Diamondback Rattlesnake (Crotalus atrox) Venom.Biomacromolecules. 2025 Jun 9;26(6):3514-3524. doi: 10.1021/acs.biomac.5c00125. Epub 2025 May 20. Biomacromolecules. 2025. PMID: 40392118 Free PMC article.
References
-
- Lee Y. C. Lee R. T. Carbohydrate–Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995;28(8):321–327. doi: 10.1021/ar00056a001. - DOI
-
- Guimond S. E. Mycroft-West C. J. Gandhi N. S. Tree J. A. Le T. T. Spalluto C. M. Humbert M. V. Buttigieg K. R. Coombes N. Elmore M. J. et al., Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike–ACE2 Interaction. ACS Cent. Sci. 2022;8(5):527–545. doi: 10.1021/acscentsci.1c01293. - DOI - PMC - PubMed
-
- Clausen T. M. Sandoval D. R. Spliid C. B. Pihl J. Painter C. D. Thacker B. E. Glass C. A. Narayanan A. Majowicz S. A. Zhang Y. et al., SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate We Show That SARS-CoV-2 Spike Protein Interacts with Both Cellular Heparan Sulfate and Angiotensin-Converting Enzyme 2 (ACE2) through Its Receptor-Binding Domain (RBD). Docking Studies Suggest a Hep. Cell. 2020;183(4):1043–1057. doi: 10.1016/j.cell.2020.09.033. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

