Dopaminergic nuclei in the chick midbrain express serotonin receptor subfamily genes
- PMID: 36425295
- PMCID: PMC9679639
- DOI: 10.3389/fphys.2022.1030621
Dopaminergic nuclei in the chick midbrain express serotonin receptor subfamily genes
Abstract
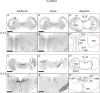
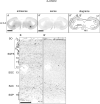
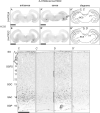
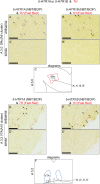
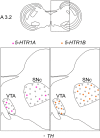
Serotonin (5-hydroxytryptamine, 5-HT) is a phylogenetically conserved modulator of numerous aspects of neural functions. Serotonergic neurons in the dorsal and median raphe nucleus provide ascending innervation to the entire forebrain and midbrain. Another important neural modulatory system exists in the midbrain, the dopaminergic system, which is associated to reward processing and motivation control. Dopaminergic neurons are distributed and clustered in the brain, classically designated as groups A8-A16. Among them, groups A8-A10 associated with reward processing and motivation control are located in the midbrain and projected to the forebrain. Recently, midbrain dopaminergic neurons were shown to be innervated by serotonergic neurons and modulated by 5-HT, with the crosstalk between serotonergic and dopaminergic systems attracting increased attention. In birds, previous studies revealed that midbrain dopaminergic neurons are located in the A8-A10 homologous clusters. However, the detailed distribution of dopaminergic neurons and the crosstalk between serotonergic and dopaminergic systems in the bird are poorly understood. To improve the understanding of the regulation of the dopaminergic by the serotonergic system, we performed in situ hybridization in the chick brainstem. We prepared RNA probes for chick orthologues of dopaminergic neuron-related genes; tyrosine hydroxylase (TH) and dopa decarboxylase (DDC), noradrenaline related genes; noradrenaline transporter (NAT) and dopamine beta-hydroxylase (DBH), and serotonin receptor genes; 5-HTR1A, 5-HTR1B, 5-HTR1D, 5-HTR1E, 5-HTR1F, 5-HTR2A, 5-HTR2B, 5-HTR2C, 5-HTR3A, 5-HTR4, 5-HTR5A, and 5-HTR7. We confirmed that the expression of tyrosine hydroxylase (TH) and NAT was well matched in all chick dopaminergic nuclei examined. This supported that the compensation of the function of dopamine transporter (DAT) by NAT is a general property of avian dopaminergic neurons. Furthermore, we showed that 5-HTR1A and 5-HTR1B were expressed in midbrain dopaminergic nuclei, suggesting the serotonergic regulation of the dopaminergic system via these receptors in chicks. Our findings will help us understand the interactions between the dopaminergic and serotonergic systems in birds at the molecular level.
Keywords: A8; chick (Gallus gallus); dopamine; optic tectum; serotonergic receptor; substantia nigra pars compacta; ventral tegmental area.
Copyright © 2022 Fujita, Aoki, Mori, Serizawa, Kihara-Negishi, Homma and Yamaguchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Serotonergic Neurons in the Chick Brainstem Express Various Serotonin Receptor Subfamily Genes.Front Physiol. 2022 Jan 17;12:815997. doi: 10.3389/fphys.2021.815997. eCollection 2021. Front Physiol. 2022. PMID: 35111079 Free PMC article.
-
Chick Hippocampal Formation Displays Subdivision- and Layer-Selective Expression Patterns of Serotonin Receptor Subfamily Genes.Front Physiol. 2022 Apr 8;13:882633. doi: 10.3389/fphys.2022.882633. eCollection 2022. Front Physiol. 2022. PMID: 35464081 Free PMC article.
-
Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat.J Comp Neurol. 1993 May 15;331(3):297-309. doi: 10.1002/cne.903310302. J Comp Neurol. 1993. PMID: 8514911
-
Ascending monoaminergic systems alterations in Alzheimer's disease. translating basic science into clinical care.Neurosci Biobehav Rev. 2013 Sep;37(8):1363-79. doi: 10.1016/j.neubiorev.2013.05.008. Epub 2013 May 24. Neurosci Biobehav Rev. 2013. PMID: 23707776 Review.
-
Reward and aversion in a heterogeneous midbrain dopamine system.Neuropharmacology. 2014 Jan;76 Pt B(0 0):351-9. doi: 10.1016/j.neuropharm.2013.03.019. Epub 2013 Apr 8. Neuropharmacology. 2014. PMID: 23578393 Free PMC article. Review.
Cited by
-
Molecular biology of serotonergic systems in avian brains.Front Mol Neurosci. 2023 Jul 19;16:1226645. doi: 10.3389/fnmol.2023.1226645. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37538316 Free PMC article. Review.
-
The Dopaminergic Cells in the Median Raphe Region Regulate Social Behavior in Male Mice.Int J Mol Sci. 2024 Apr 13;25(8):4315. doi: 10.3390/ijms25084315. Int J Mol Sci. 2024. PMID: 38673899 Free PMC article.
-
Molecular characterization of chicken DA systems reveals that the avian personality gene, DRD4, is expressed in the mitral cells of the olfactory bulb.Front Neuroanat. 2025 Jan 15;19:1531200. doi: 10.3389/fnana.2025.1531200. eCollection 2025. Front Neuroanat. 2025. PMID: 39886560 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

