Identification of a metastable uranium metal-organic framework isomer through non-equilibrium synthesis
- PMID: 36425512
- PMCID: PMC9667927
- DOI: 10.1039/d2sc04783g
Identification of a metastable uranium metal-organic framework isomer through non-equilibrium synthesis
Abstract
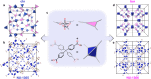
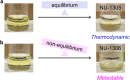
Since the structure of supramolecular isomers determines their performance, rational synthesis of a specific isomer hinges on understanding the energetic relationships between isomeric possibilities. To this end, we have systematically interrogated a pair of uranium-based metal-organic framework topological isomers both synthetically and through density functional theory (DFT) energetic calculations. Although synthetic and energetic data initially appeared to mismatch, we assigned this phenomenon to the appearance of a metastable isomer, driven by levers defined by Le Châtelier's principle. Identifying the relationship between structure and energetics in this study reveals how non-equilibrium synthetic conditions can be used as a strategy to target metastable MOFs. Additionally, this study demonstrates how defined MOF design rules may enable access to products within the energetic phase space which are more complex than conventional binary (e.g., kinetic vs. thermodynamic) products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
O. K. F. has a financial interest in NuMat Technologies, a startup company that is seeking to commercialize MOFs. All other authors declare no competing interests.
Figures



References
-
- Yang X. Coulombe-Huntington J. Kang S. Sheynkman G. M. Hao T. Richardson A. Sun S. Yang F. Shen Y. A. Murray R. R. Spirohn K. Begg B. E. Duran-Frigola M. MacWilliams A. Pevzner S. J. Zhong Q. Trigg S. A. Tam S. Ghamsari L. Sahni N. Yi S. Rodriguez M. D. Balcha D. Tan G. Costanzo M. Andrews B. Boone C. Zhou X. J. Salehi-Ashtiani K. Charloteaux B. Chen A. A. Calderwood M. A. Aloy P. Roth F. P. Hill D. E. Iakoucheva L. M. Xia Y. Vidal M. Cell. 2016;164:805–817. doi: 10.1016/j.cell.2016.01.029. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

