The effect of Ni gella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: A randomized controlled clinical trial
- PMID: 36425571
- PMCID: PMC9681154
- DOI: 10.3389/fphar.2022.1011522
The effect of Ni gella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: A randomized controlled clinical trial
Abstract
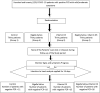
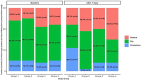
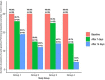
Background: The coronavirus disease 2019 (COVID-19) is a novel coronavirus that causes severe infection in the respiratory system. Since the immune status plays an essential role in combating COVID-19, herbal medicines, which have an immunomodulatory effect, may help prevent and even treat COVID-19. Nigella sativa is one of the herbal medicines with antiviral and immunomodulatory activities, and its therapeutic effectiveness makes it a promising add-on therapy for COVID-19. In addition, vitamin D3 has an immunomodulatory role, but the effect of therapeutic vitamin D3 supplementation in SARS-CoV-2 infection is still not well-known. Objective: This study aims to investigate the effects of Nigella sativa and vitamin D3 as single supplemental therapies and in combination on viral clearance indicated by a negative polymerase chain reaction and the alleviation of symptoms during the study follow-up duration of 14 days. Patients and Methods: The study design was an open-label randomized controlled clinical trial conducted at the Respiratory Hospital at the Kobry El Qobba Armed Forces Medical Complex. In total, 120 COVID-19 patients with mild to moderate symptoms were randomly assigned to four groups, with thirty patients each, as follows: Group 1 received an oral dose of 900 mg Nigella sativa through 450 mg soft gelatin capsules twice daily for two weeks; Group 2 received 2,000 IU of vitamin D3 through 1000-IU tablets given as two tablets, once daily; Group 3 received 900 mg of Nigella sativa and 2,000 IU of vitamin D3 in the same manner of dosing as in the previous groups; and Group 4 was the control group. All groups received standard therapy for COVID-19 infections and clinical management of COVID-19's clinical symptoms. Results: The Nigella sativa-vitamin D3 combination in addition to the standard therapy for COVID-19 infections significantly contributed to the alleviation of most COVID-19 symptoms: 50% of patients were free of cough after 7 days, 70% showed an absence of fatigue after 4 days, 80% had no headache after 5 days, 90% were free of rhinorrhea after 7 days, and 86.7% of the patients had no dyspnea after 7 days. Moreover, patients in the four studied groups showed a reduced median temperature after 3 days of treatment. Negative results of the polymerase chain reaction (PCR) test recorded on the 7th and 14th day of therapy were superior in the Nigella sativa and vitamin D3 combination arm compared to those of the other studied arms where the value of the odds ratio (OR) on the 7th day was 0.13 with 95% CI: 0.03-0.45 and that of the 14th day was 0.09 with 95% CI: 0.02-0.3. Conclusion: The results of this study showed a promising therapeutic benefit of the administration of Nigella sativa and vitamin D3 combination in COVID-19 patients with mild to moderate symptoms. Additionally, the remarkable viral clearance in a short time interval and reduction in the severity and progression of symptoms recommended the use of this combination as an add-on therapy for the management of COVID-19 patients. Clinical Trial Registration: ClinicalTrials.gov, Identifier: NCT04981743.
Keywords: COVID-19; Nigella sativa; add-on therapy; complementary medicine; immunomodulation; vitamin D3.
Copyright © 2022 Said, Abdulbaset, El-Kholy, Besckales and Sabri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial.Front Pharmacol. 2022 Jun 7;13:898062. doi: 10.3389/fphar.2022.898062. eCollection 2022. Front Pharmacol. 2022. PMID: 35747751 Free PMC article.
-
Vitamin D supplementation for the treatment of COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. Cochrane Database Syst Rev. 2021. PMID: 34029377 Free PMC article.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
Cited by
-
Herbal medicine approach to relieving dyspnea: A narrative review of efficacy and mechanisms.Iran J Basic Med Sci. 2025;28(9):1140-1162. doi: 10.22038/ijbms.2025.85518.18486. Iran J Basic Med Sci. 2025. PMID: 40809176 Free PMC article. Review.
-
Therapeutic effects of vitamin D supplementation on COVID-19 aggravation: a systematic review and meta-analysis of randomized controlled trials.Front Pharmacol. 2024 May 27;15:1367686. doi: 10.3389/fphar.2024.1367686. eCollection 2024. Front Pharmacol. 2024. PMID: 38860175 Free PMC article.
-
A narrative review focusing on randomized clinical trials of vitamin D supplementation for COVID-19 disease.Front Nutr. 2025 Jan 7;11:1461485. doi: 10.3389/fnut.2024.1461485. eCollection 2024. Front Nutr. 2025. PMID: 39839285 Free PMC article. Review.
-
NLRP3 Inflammasomes: Dual Function in Infectious Diseases.J Immunol. 2024 Aug 15;213(4):407-417. doi: 10.4049/jimmunol.2300745. J Immunol. 2024. PMID: 39102612 Free PMC article. Review.
-
Nigella sativa and its chemical constituents: pre-clinical and clinical evidence for their potential anti-SARS-CoV-2 effects.Inflammopharmacology. 2024 Feb;32(1):273-285. doi: 10.1007/s10787-023-01385-9. Epub 2023 Nov 15. Inflammopharmacology. 2024. PMID: 37966624 Review.
References
-
- Abdallah H. M., El-Halawany A. M., Darwish K. M., Algandaby M. M., Mohamed G. A., Ibrahim S. R. M., et al. (2022). Bio-guided isolation of SARS-CoV-2 main protease inhibitors from medicinal plants: In vitro assay and molecular dynamics. Plants 11 (15), 1914. 10.3390/PLANTS11151914 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

