Cis- trans isomerization of dimethyl 2,3-dibromofumarate
- PMID: 36425717
- PMCID: PMC9661881
- DOI: 10.1039/d2ra05996g
Cis- trans isomerization of dimethyl 2,3-dibromofumarate
Abstract
The isomerization of dimethyl 2,3-dibromofumarate in chloroform solutions was investigated by the combination of nuclear magnetic resonance (NMR) and density functional theory (DFT) calculations. The bromination of dimethyl acetylenedicarboxylate leading to dimethyl 2,3-dibromofumarate produces the trans isomer initially, which however converts into the more stable cis isomer. The conversion from trans to cis is spontaneous and greatly accelerated by light.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


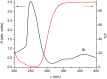
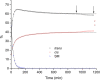
References
-
- Diederich F. Schreiher M. Anthony J. Suter U. W. Colussi M. Nesper R. Spahr M. E. Gunter P. Bosshard C. Kaatz P. Wachter P. Degiorgi L. Bommeli F. Hubrich M. Gross M. Boudon C. Gisselbrecht J.-P. Adv. Mater. 1994;6:786–790. doi: 10.1002/adma.19940061017. - DOI
LinkOut - more resources
Full Text Sources

