Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications
- PMID: 36425787
- PMCID: PMC9679376
- DOI: 10.3389/fcimb.2022.997018
Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications
Abstract
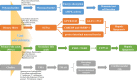
The incidence of nonalcoholic fatty liver disease (NAFLD) is increasing recently and has become one of the most common clinical liver diseases. Since the pathogenesis of NAFLD has not been completely elucidated, few effective therapeutic drugs are available. As the "second genome" of human body, gut microbiota plays an important role in the digestion, absorption and metabolism of food and drugs. Gut microbiota can act as an important driver to advance the occurrence and development of NAFLD, and to accelerate its progression to cirrhosis and hepatocellular carcinoma. Growing evidence has demonstrated that gut microbiota and its metabolites directly affect intestinal morphology and immune response, resulting in the abnormal activation of inflammation and intestinal endotoxemia; gut dysbiosis also causes dysfunction of gut-liver axis via alteration of bile acid metabolism pathway. Because of its composition diversity and disease-specific expression characteristics, gut microbiota holds strong promise as novel biomarkers and therapeutic targets for NAFLD. Intervening intestinal microbiota, such as antibiotic/probiotic treatment and fecal transplantation, has been a novel strategy for preventing and treating NAFLD. In this article, we have reviewed the emerging functions and association of gut bacterial components in different stages of NAFLD progression and discussed its potential implications in NAFLD diagnosis and therapy.
Keywords: bile acid metabolism; gut dysbiosis; nonalcoholic fatty liver disease; novel treatment strategies; probiotics.
Copyright © 2022 Fang, Yu, Li, Yao, Fang, Yoon and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Arelaki S., Koletsa T., Sinakos E., Papadopoulos V., Arvanitakis K., Skendros P., et al. . (2022). Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 481 (3), 455–465. doi: 10.1007/S00428-022-03330-7 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

