Prediction of immunotherapy efficacy and immunomodulatory role of hypoxia in colorectal cancer
- PMID: 36425871
- PMCID: PMC9679351
- DOI: 10.1177/17588359221138383
Prediction of immunotherapy efficacy and immunomodulatory role of hypoxia in colorectal cancer
Abstract
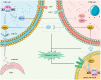
Immunotherapy has been used in the clinical treatment of colorectal cancer (CRC); however, most patients fail to achieve satisfactory survival benefits. Biomarkers with high specificity and sensitivity are being increasingly developed to predict the efficacy of CRC immunotherapy. In addition to DNA alteration markers, such as microsatellite instability/mismatch repair and tumor mutational burden, immune cell infiltration and immune checkpoints (ICs), epigenetic changes and no-coding RNA, and gut microbiomes all show potential predictive ability. Recently, the hypoxic tumor microenvironment (TME) has been identified as a key factor mediating CRC immune evasion and resistance to treatment. Hypoxia-inducible factor-1α is the central transcription factor in the hypoxia response that drives the expression of a vast number of survival genes by binding to the hypoxia response element in cancer and immune cells in the TME. Hypoxia regulates angiogenesis, immune cell infiltration and activation, expression of ICs, and secretion of various immune molecules in the TME and is closely associated with the immunotherapeutic efficacy of CRC. Currently, various agents targeting hypoxia have been found to improve the TME and enhance the efficacy of immunotherapy. We reviewed current markers commonly used in CRC to predict therapeutic efficacy and the mechanisms underlying hypoxia-induced angiogenesis and tumor immune evasion. Exploring the mechanisms by which hypoxia affects the TME will assist the discovery of new immunotherapeutic predictive biomarkers and development of more effective combinations of agents targeting hypoxia and immunotherapy.
Keywords: colorectal cancer; hypoxia; immune checkpoint inhibitors; immunotherapy; tumor microenvironment.
© The Author(s), 2022.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




Similar articles
-
Construction and validation of a novel angiogenesis pattern to predict prognosis and immunotherapy efficacy in colorectal cancer.Aging (Albany NY). 2023 Nov 7;15(21):12413-12450. doi: 10.18632/aging.205189. Epub 2023 Nov 7. Aging (Albany NY). 2023. PMID: 37938164 Free PMC article.
-
Identification of Hypoxia-Related Subtypes, Establishment of Prognostic Models, and Characteristics of Tumor Microenvironment Infiltration in Colon Cancer.Front Genet. 2022 Jun 17;13:919389. doi: 10.3389/fgene.2022.919389. eCollection 2022. Front Genet. 2022. PMID: 35783281 Free PMC article.
-
Immunotherapy in colorectal cancer.Adv Cancer Res. 2021;151:137-196. doi: 10.1016/bs.acr.2021.03.002. Epub 2021 Jun 7. Adv Cancer Res. 2021. PMID: 34148613 Review.
-
The regulation of immune checkpoints by the hypoxic tumor microenvironment.PeerJ. 2021 May 7;9:e11306. doi: 10.7717/peerj.11306. eCollection 2021. PeerJ. 2021. PMID: 34012727 Free PMC article.
-
Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy.J Exp Clin Cancer Res. 2021 Jan 9;40(1):24. doi: 10.1186/s13046-020-01820-7. J Exp Clin Cancer Res. 2021. PMID: 33422072 Free PMC article. Review.
Cited by
-
Anti-angiogenesis in colorectal cancer therapy.Cancer Sci. 2024 Mar;115(3):734-751. doi: 10.1111/cas.16063. Epub 2024 Jan 17. Cancer Sci. 2024. PMID: 38233340 Free PMC article. Review.
-
Identification of a Novel Oxidative Stress- and Anoikis-Related Prognostic Signature and Its Immune Landscape Analysis in Non-Small Cell Lung Cancer.Int J Mol Sci. 2023 Nov 10;24(22):16188. doi: 10.3390/ijms242216188. Int J Mol Sci. 2023. PMID: 38003378 Free PMC article.
-
Targeting lncRNAs of colorectal cancers with natural products.Front Pharmacol. 2023 Jan 9;13:1050032. doi: 10.3389/fphar.2022.1050032. eCollection 2022. Front Pharmacol. 2023. PMID: 36699052 Free PMC article. Review.
-
Modulation of host N6-methyladenosine modification by gut microbiota in colorectal cancer.World J Gastroenterol. 2024 Oct 14;30(38):4175-4193. doi: 10.3748/wjg.v30.i38.4175. World J Gastroenterol. 2024. PMID: 39493326 Free PMC article. Review.
-
Prognostic Features and Potential for Immune Therapy in Metastatic Mismatch Repair-Deficient Colorectal Cancer: A Retrospective Analysis of a Large Consecutive Population-Based Patient Series.Cancer Med. 2025 Jan;14(1):e70555. doi: 10.1002/cam4.70555. Cancer Med. 2025. PMID: 39783790 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Jiang BH, Rue E, Wang GL, et al.. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 1996; 271: 17771–17778. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

