A single birth dose of Hepatitis B vaccine induces polyfunctional CD4+ T helper cells
- PMID: 36426360
- PMCID: PMC9681035
- DOI: 10.3389/fimmu.2022.1043375
A single birth dose of Hepatitis B vaccine induces polyfunctional CD4+ T helper cells
Abstract
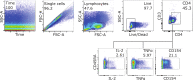
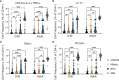
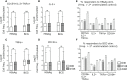
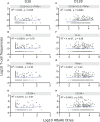
A single birth-dose of Hepatitis B vaccine (HepB) can protect newborns from acquiring Hepatitis B infection through vertical transmission, though several follow-up doses are required to induce long-lived protection. In addition to stimulating antibodies, a birth-dose of HepB might also induce polyfunctional CD4+ T-cells, which may contribute to initial protection. We investigated whether vaccination with HepB in the first week of life induced detectable antigen-specific CD4+ T-cells after only a single dose and following completion of the entire HepB vaccine schedule (3 doses). Using HBsAg- stimulated peripheral blood mononuclear cells from 344 infants, we detected increased populations of antigen-specific polyfunctional CD154+IL-2+TNFα+ CD4+ T-cells following a single birth-dose of HepB in a proportion of infants. Frequencies of polyfunctional T-cells increased following the completion of the HepB schedule but increases in the proportion of responders as compared to following only one dose was marginal. Polyfunctional T-cells correlated positively with serum antibody titres following the birth dose (day30) and completion of the 3-dose primary HepB vaccine series (day 128). These data indicate that a single birth dose of HepB provides immune priming for both antigen-specific B- and T cells.
Keywords: BCG; CD154; CD4 + T helper cell; Hepatitis B vaccine; T-cell activation; antibody titres; neonate.
Copyright © 2022 Strandmark, Darboe, Diray-Arce, Ben-Othman, Vignolo, Rao, Smolen, Leroux-Roels, Idoko, Sanchez-Schmitz, Ozonoff, Levy, Kollmann, Marchant and Kampmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

