Oropharyngeal tumor cells induce COX-2 expression in peripheral blood monocytes by secretion of IL-1α
- PMID: 36426368
- PMCID: PMC9681149
- DOI: 10.3389/fimmu.2022.1011772
Oropharyngeal tumor cells induce COX-2 expression in peripheral blood monocytes by secretion of IL-1α
Abstract
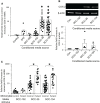
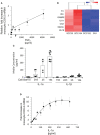
Oropharyngeal squamous cell cancer (OPC) accounts for 3% of all cancers and greater than 1.5% of all cancer deaths in the United States, with marked treatment-associated morbidity in survivors. More than 80% of OPC is caused by HPV16. Tumors induced by HPV have been linked to impaired immune functions, with most studies focused on the local tumor microenvironment. Fewer studies have characterized the effects of these tumors on systemic responses in OPC, especially innate responses that drive subsequent adaptive responses, potentially creating feed-back loops favorable to the tumor. Here we report that elevated plasma levels of PGE2 are expressed in half of patients with OPC secondary to overexpression of COX-2 by peripheral blood monocytes, and this expression is driven by IL-1α secreted by the tumors. Monocytes from patients are much more sensitive to the stimulation than monocytes from controls, suggesting the possibility of enhanced immune-modulating feed-back loops. Furthermore, control monocytes pre-exposed to PGE2 overexpress COX-2 in response to IL-1α, simulating responses made by monocytes from some OPC patients. Disrupting the PGE2/IL-1α feed-back loop can have potential impact on targeted medical therapies.
Keywords: COX-2; IL-1 alpha; PGE 2; monocytes; oropharyngeal cancer.
Copyright © 2022 DeVoti, Israr, Lam, Papayannakos, Frank, Kamdar, Pereira, Abramson, Steinberg and Bonagura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Oropharyngeal carcinomas induce circulating monocytes to express a TAM-like pro-tumor expression profile that suppresses T-cell proliferation.Front Immunol. 2025 Mar 19;16:1539780. doi: 10.3389/fimmu.2025.1539780. eCollection 2025. Front Immunol. 2025. PMID: 40176808 Free PMC article.
-
Areca nut extracts increased the expression of cyclooxygenase-2, prostaglandin E2 and interleukin-1α in human immune cells via oxidative stress.Arch Oral Biol. 2013 Oct;58(10):1523-31. doi: 10.1016/j.archoralbio.2013.05.006. Epub 2013 Aug 3. Arch Oral Biol. 2013. PMID: 23916228
-
Cyclooxygenase regulates human oropharyngeal carcinomas via the proinflammatory cytokine IL-6: a general role for inflammation?FASEB J. 2000 Aug;14(11):1499-507. doi: 10.1096/fj.14.11.1499. FASEB J. 2000. PMID: 10928984
-
The IL-1/IL-1R axis induces greater fibroblast-derived chemokine release in human papillomavirus-negative compared to positive oropharyngeal cancer.Int J Cancer. 2019 Jan 15;144(2):334-344. doi: 10.1002/ijc.31852. Epub 2018 Nov 26. Int J Cancer. 2019. PMID: 30191960 Free PMC article.
-
Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.Cancer Sci. 2016 Apr;107(4):391-7. doi: 10.1111/cas.12901. Epub 2016 Mar 18. Cancer Sci. 2016. PMID: 27079437 Free PMC article. Review.
Cited by
-
Reprogramming inflammation: Mechanisms and therapeutic targeting of eicosanoids and pro-resolving mediators.Eur J Pharmacol. 2025 Sep 15;1003:177924. doi: 10.1016/j.ejphar.2025.177924. Epub 2025 Jul 5. Eur J Pharmacol. 2025. PMID: 40623536 Review.
-
Oropharyngeal carcinomas induce circulating monocytes to express a TAM-like pro-tumor expression profile that suppresses T-cell proliferation.Front Immunol. 2025 Mar 19;16:1539780. doi: 10.3389/fimmu.2025.1539780. eCollection 2025. Front Immunol. 2025. PMID: 40176808 Free PMC article.
References
-
- Mehta P, Tolar J. Fanconi anemia. GeneReviews (2002) 19:1–15.
-
- Bonagura VR, Du Z, Ashouri E, Luo L, Hatam LJ, Devoti JA, et al. . Activating killer cell immunoglobulin-like receptors 3DS1 and 2DS1 protect against developing the severe form of recurrent respiratory papillomatosis. Hum Immunol (2010) 71:212–9. doi: 10.1016/j.humimm.2009.10.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

