Liposomal amphotericin B-the past
- PMID: 36426673
- PMCID: PMC9693798
- DOI: 10.1093/jac/dkac351
Liposomal amphotericin B-the past
Abstract
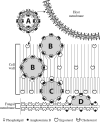
The discovery of amphotericin B, a polyene antifungal compound, in the 1950s, and the formulation of this compound in a liposomal drug delivery system, has resulted in decades of use in systemic fungal infections. The use of liposomal amphotericin B formulation is referenced in many international guidelines for the treatment of fungal infections such as Aspergillus and cryptococcal disease and Candida infections, as well as other less common infections such as visceral leishmaniasis. With the development of liposomal amphotericin B, an improved therapeutic index could be achieved that allowed the attainment of higher drug concentrations in both the plasma and tissue while simultaneously lowering the toxicity compared with amphotericin B deoxycholate. In over 30 years of experience with this drug, a vast amount of information has been collected on preclinical and clinical efficacy against a wide variety of pathogens, as well as evidence on its toxicity. This article explores the history and nature of the liposomal formulation, the key clinical studies that developed the pharmacokinetic, safety and efficacy profile of the liposomal formulation, and the available microbiological data.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- Homei A, Worboys M. Fungal Disease in Britain and the United States 1850–2000: Mycoses and Modernity. Palgrave Macmillan, 2013. - PubMed
-
- de Kruijff B, Gerritsen WJ, Oerlemans Aet al. . Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta 1974; 339: 30–43. 10.1016/0005-2736(74)90330-7 - DOI - PubMed
-
- Electronic Medicines Compendium . Fungizone 50 mg powder for sterile concentrate. Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/10716/smpc#gref.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

