Depletion attractions drive bacterial capture on both non-fouling and adhesive surfaces, enhancing cell orientation
- PMID: 36426747
- PMCID: PMC9837788
- DOI: 10.1039/d2sm01248k
Depletion attractions drive bacterial capture on both non-fouling and adhesive surfaces, enhancing cell orientation
Abstract
Depletion attractions, occurring between surfaces immersed in a polymer solution, drive bacteria adhesion to a variety of surfaces. The latter include the surfaces of non-fouling coatings such as hydrated polyethylene glycol (PEG) layers but also, as demonstrated in this work, surfaces that are bacteria-adhesive, such as that of glass. Employing a flagella free E. coli strain, we demonstrate that cell adhesion on glass is enhanced by dissolved polyethylene oxide (PEO), exhibiting a faster rate and greater numbers of captured cells compared with the slower capture of the same cells on glass from a buffer solution. After removal of depletant, any cell retention appears to be governed by the substrate, with cells immediately released from non-fouling PEG surfaces but retained on glass. A distinguishing feature of cells captured by depletion on PEG surfaces is their orientation parallel to the surface and very strong alignment with flow. This suggests that, in the moments of capture, cells are able to rotate as they adhere. By contrast on glass, captured cells are substantially more upright and less aligned by flow. On glass the free polymer exerts forces that slightly tip cells towards the surface. Free polymer also holds cells still on adhesive and non-fouling surfaces alike but, upon removal of free PEO, cells retained on glass tend to be held by one end and exhibit a Brownian type rotational rocking.
Figures

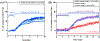



References
-
- Asakura S and Oosawa F, On Interactions between 2 Bodies Immersed in a Solution of Macromolecules, J. Chem. Phys, 1954, 22, 1255–1256.
-
- Asakura S and Oosawa F, Interaction between Particles Suspended in Solutions of Macromolecules, Journal of Polymer Science, 1958, 33, 183–192.
-
- Joanny JF, Leibler L and DeGennes PG, Effects of Polymer Solutions on Colloid Stability, J. Polym. Sci. Polym Phys, 1979, 17, 1073–1084.
-
- Dijkstra M, van Roij R and Evans R, Phase Diagram of Highly Asymmetric Binary Hard-Sphere Mixtures, Physical Review E, 1999, 59, 5744–5771. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

