Detecting Early-Stage Cohesion Due to Calcium Silicate Hydration with Rheology and Surface Force Apparatus
- PMID: 36426749
- PMCID: PMC9730907
- DOI: 10.1021/acs.langmuir.2c02783
Detecting Early-Stage Cohesion Due to Calcium Silicate Hydration with Rheology and Surface Force Apparatus
Abstract
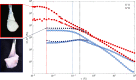
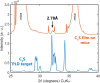
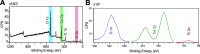
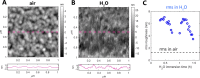
Extremely robust cohesion triggered by calcium silicate hydrate (C-S-H) precipitation during cement hardening makes concrete one of the most commonly used man-made materials. Here, in this proof-of-concept study, we seek an additional nanoscale understanding of early-stage cohesive forces acting between hydrating model tricalcium silicate (C3S) surfaces by combining rheological and surface force measurements. We first used time-resolved small oscillatory rheology measurements (SAOSs) to characterize the early-stage evolution of the cohesive properties of a C3S paste and a C-S-H gel. SAOS revealed the reactive and viscoelastic nature of C3S pastes, in contrast with the nonreactive but still viscoelastic nature of the C-S-H gel, which proves a temporal variation in the cohesion during microstructural physicochemical rearrangements in the C3S paste. We further prepared thin films of C3S by plasma laser deposition (PLD) and demonstrated that these films are suitable for force measurements in the surface force apparatus (SFA). We measured surface forces acting between two thin C3S films exposed to water and subsequent in situ calcium silicate hydrate precipitation. With the SFA and SFA-coupled interferometric measurements, we resolved that C3S surface reprecipitation in water was associated with both increasing film thickness and progressively stronger adhesion (pull-off force). The lasting adhesion developing between the growing surfaces depended on the applied load, pull-off rate, and time in contact. These properties indicated the viscoelastic character of the soft, gel-like reprecipitated layer, pointing to the formation of C-S-H. Our findings confirm the strong cohesive properties of hydrated calcium silicate surfaces that, based on our preliminary SFA measurements, are attributed to sharp changes in the surface microstructure. In contact with water, the brittle and rough C3S surfaces with little contact area weather into soft, gel-like C-S-H nanoparticles with a much larger surface area available for forming direct contacts between interacting surfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Taylor H. F.et al.Cement Chemistry: Portland Cement and Its Major Constituent Phases; Thomas Telford Ltd.: London, U.K., 1997; Vol. 2, pp 1–28.
-
- Gartner E. M.; Young J. F.; Damidot D. A.; Jawed I.. Hydration of Portland Cement. In Structure and Performance of Cements; Spon Press: London, 2002; Vol. 2, pp 57–113.
-
- Valentini L.; Dalconi M. C.; Favero M.; Artioli G.; Ferrari G. In-Situ XRD Measurement and Quantitative Analysis of Hydrating Cement: Implications for Sulfate Incorporation in C–S–H. J. Am. Ceram. Soc. 2015, 98, 1259–1264. 10.1111/jace.13401. - DOI
-
- Thomas J. J. A new approach to modeling the nucleation and growth kinetics of tricalcium silicate hydration. J. Am. Ceram. Soc. 2007, 90, 3282–3288. 10.1111/j.1551-2916.2007.01858.x. - DOI
LinkOut - more resources
Full Text Sources

