Prediction of tissue-of-origin of early stage cancers using serum miRNomes
- PMID: 36426871
- PMCID: PMC9825310
- DOI: 10.1093/jncics/pkac080
Prediction of tissue-of-origin of early stage cancers using serum miRNomes
Abstract
Background: Noninvasive detection of early stage cancers with accurate prediction of tumor tissue-of-origin could improve patient prognosis. Because miRNA profiles differ between organs, circulating miRNomics represent a promising method for early detection of cancers, but this has not been shown conclusively.
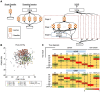
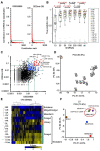
Methods: A serum miRNA profile (miRNomes)-based classifier was evaluated for its ability to discriminate cancer types using advanced machine learning. The training set comprised 7931 serum samples from patients with 13 types of solid cancers and 5013 noncancer samples. The validation set consisted of 1990 cancer and 1256 noncancer samples. The contribution of each miRNA to the cancer-type classification was evaluated, and those with a high contribution were identified.
Results: Cancer type was predicted with an accuracy of 0.88 (95% confidence interval [CI] = 0.87 to 0.90) in all stages and an accuracy of 0.90 (95% CI = 0.88 to 0.91) in resectable stages (stages 0-II). The F1 score for the discrimination of the 13 cancer types was 0.93. Optimal classification performance was achieved with at least 100 miRNAs that contributed the strongest to accurate prediction of cancer type. Assessment of tissue expression patterns of these miRNAs suggested that miRNAs secreted from the tumor environment could be used to establish cancer type-specific serum miRNomes.
Conclusions: This study demonstrates that large-scale serum miRNomics in combination with machine learning could lead to the development of a blood-based cancer classification system. Further investigations of the regulating mechanisms of the miRNAs that contributed strongly to accurate prediction of cancer type could pave the way for the clinical use of circulating miRNA diagnostics.
© The Author(s) 2022. Published by Oxford University Press.
Figures




Comment in
-
Multicancer early detection tests: where are we?JNCI Cancer Spectr. 2023 Jan 3;7(1):pkac084. doi: 10.1093/jncics/pkac084. JNCI Cancer Spectr. 2023. PMID: 36453871 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
