Reduced T Cell Priming in Microbially Experienced "Dirty" Mice Results from Limited IL-27 Production by XCR1+ Dendritic Cells
- PMID: 36426978
- PMCID: PMC10065988
- DOI: 10.4049/jimmunol.2200324
Reduced T Cell Priming in Microbially Experienced "Dirty" Mice Results from Limited IL-27 Production by XCR1+ Dendritic Cells
Abstract
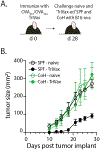
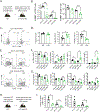
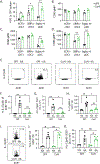
Successful vaccination strategies offer the potential for lifelong immunity against infectious diseases and cancer. There has been increased attention regarding the limited translation of some preclinical findings generated using specific pathogen-free (SPF) laboratory mice to humans. One potential reason for the difference between preclinical and clinical findings lies in maturation status of the immune system at the time of challenge. In this study, we used a "dirty" mouse model, where SPF laboratory mice were cohoused (CoH) with pet store mice to permit microbe transfer and immune system maturation, to investigate the priming of a naive T cell response after vaccination with a peptide subunit mixed with polyinosinic-polycytidylic acid and agonistic anti-CD40 mAb. Although this vaccination platform induced robust antitumor immunity in SPF mice, it failed to do so in microbially experienced CoH mice. Subsequent investigation revealed that despite similar numbers of Ag-specific naive CD4 and CD8 T cell precursors, the expansion, differentiation, and recall responses of these CD4 and CD8 T cell populations in CoH mice were significantly reduced compared with SPF mice after vaccination. Evaluation of the dendritic cell compartment revealed reduced IL-27p28 expression by XCR1+ dendritic cells from CoH mice after vaccination, correlating with reduced T cell expansion. Importantly, administration of recombinant IL-27:EBI3 complex to CoH mice shortly after vaccination significantly boosted Ag-specific CD8 and CD4 T cell expansion, further implicating the defect to be T cell extrinsic. Collectively, our data show the potential limitation of exclusive use of SPF mice when testing vaccine efficacy.
Copyright © 2022 by The American Association of Immunologists, Inc.
Figures


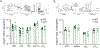

Similar articles
-
Functional Specialty of CD40 and Dendritic Cell Surface Lectins for Exogenous Antigen Presentation to CD8(+) and CD4(+) T Cells.EBioMedicine. 2016 Jan 28;5:46-58. doi: 10.1016/j.ebiom.2016.01.029. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077111 Free PMC article.
-
CD4-directed peptide vaccination augments an antitumor response, but efficacy is limited by the number of CD8+ T cell precursors.J Immunol. 2004 Apr 1;172(7):4215-24. doi: 10.4049/jimmunol.172.7.4215. J Immunol. 2004. PMID: 15034034
-
Differential requirements of T cell subsets for CD40 costimulation in immunity to Blastomyces dermatitidis.J Immunol. 2006 May 1;176(9):5538-47. doi: 10.4049/jimmunol.176.9.5538. J Immunol. 2006. PMID: 16622023
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
CD115+ monocytes protect microbially experienced mice against E. coli-induced sepsis.Cell Rep. 2023 Nov 28;42(11):113345. doi: 10.1016/j.celrep.2023.113345. Epub 2023 Oct 28. Cell Rep. 2023. PMID: 38111515 Free PMC article.
-
Integrating natural commensals and pathogens into preclinical mouse models.Nat Rev Immunol. 2025 May;25(5):385-397. doi: 10.1038/s41577-024-01108-3. Epub 2024 Nov 19. Nat Rev Immunol. 2025. PMID: 39562646 Free PMC article. Review.
-
Wild-Mouse-Derived Gut Microbiome Transplantation in Laboratory Mice Partly Alleviates House-Dust-Mite-Induced Allergic Airway Inflammation.Microorganisms. 2024 Dec 4;12(12):2499. doi: 10.3390/microorganisms12122499. Microorganisms. 2024. PMID: 39770703 Free PMC article.
-
Physiological microbial exposure normalizes memory T cell surveillance of the brain and modifies host seizure outcomes.Nat Immunol. 2025 Jul;26(7):1087-1098. doi: 10.1038/s41590-025-02174-y. Epub 2025 Jun 13. Nat Immunol. 2025. PMID: 40514419
References
-
- Hand TW, and Kaech SM. 2009. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol. Res 45: 46–61. - PubMed
-
- Mueller SN, Gebhardt T, Carbone FR, and Heath WR. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol 31: 137–161. - PubMed
-
- Ma M, Liu J, Jin S, and Wang L. 2020. Development of tumour peptide vaccines: From universalization to personalization. Scand. J. Immunol 91: e12875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

