The efficacy and safety of caplacizumab in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura: an open-label phase 2/3 study
- PMID: 36427162
- PMCID: PMC9970947
- DOI: 10.1007/s12185-022-03495-6
The efficacy and safety of caplacizumab in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura: an open-label phase 2/3 study
Abstract
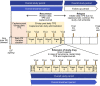
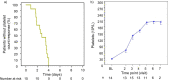
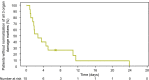
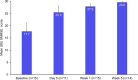
Caplacizumab is an anti-von Willebrand factor humanized single-variable-domain immunoglobulin fragment whose efficacy and safety in immune-mediated thrombotic thrombocytopenia purpura (iTTP) have been demonstrated in international studies. This prospective, open-label phase 2/3 study evaluated caplacizumab 10 mg administered daily during plasma exchange and for 30 days afterward, in combination with immunosuppressive treatment, in Japanese adults with a clinical diagnosis of iTTP (new or recurrent). The primary endpoint was prevention of iTTP recurrence; key secondary endpoints included time to platelet count response, time to organ damage normalization, and safety. Among 21 treated patients, 1 of 15 (6.7%) evaluable patients developed iTTP recurrence. Median time to normalization was 2.79 days for platelet count and 2.65 days for organ damage markers (n = 15). Treatment-emergent adverse events (TEAEs) were mostly mild to moderate in severity; the most frequently reported caplacizumab-related TEAEs were increased alanine aminotransferase, epistaxis, and gastrointestinal hemorrhage (all in 9.5% of patients). At least one bleeding event was reported in 7 of 21 patients (33%). Caplacizumab was effective in Japanese patients with iTTP, with a low rate of iTTP recurrence, rapid normalization of platelet counts and organ damage markers, and no unexpected TEAEs. Trial registration: ClinicalTrials.gov identifier, NCT04074187.
Keywords: ADAMTS13; Caplacizumab; Single-domain antibody; Thrombotic thrombocytopenic purpura; Von Willebrand factor inhibitor.
© 2022. The Author(s).
Conflict of interest statement
YM served as a consultant for Sanofi K.K. and Zenyakukogyo Co., Ltd., has participated in advisory boards for Sanofi K.K., and received research funding from Sanofi K.K. KI received honoraria from Celgene Co., Ltd., Bristol-Myers Squibb K.K., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Otuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K, Meiji Seika Pharma Co. Ltd., Ono Pharmaceutical Co. Ltd., Janssen Pharmaceutical K.K., Nippon Shinyaku Co., Ltd., and AstraZeneca K.K. SI received honoraria from Sanofi K.K., AstraZeneca K.K, and Chugai Pharmaceutical Co., Ltd. TM served on advisory boards for Takeda Pharmaceutical Co. Ltd. (Baxalta/Shire), Bayer Yakuhin, Ltd., Novo Nordisk Pharma Ltd., Chugai Pharmaceutical Co., Ltd., and Pfizer Inc., received educational and investigational support from Chugai Pharmaceutical Co., Ltd. and Novo Nordisk Pharma Ltd., and received honoraria from Takeda Pharmaceutical Co. Ltd. (Shire), Bayer Yakuhin, Ltd., Sanofi K.K. (Bioverative), Chugai Pharmaceutical Co., Ltd., CSL Behring K.K., Japan Blood Products Organization, KM Biologics Co., Ltd., Kyowa Kirin Co., Ltd., Nichiyaku, Novo Nordisk Pharma Ltd., Octapharma AG, and Sysmex Corporation. MM received honoraria from Asahi Kasei Pharma Corporation, Chugai Pharmaceutical Co., Ltd., and Alexion Pharma Inc., has served as a consultant for Sanofi K.K., Takeda Pharmaceutical Co. Ltd., and Alexion Pharma Inc., received research funding from Asahi Kasei Pharma Corporation and Chugai Pharmaceutical Co., Ltd., and received patents and royalties from Alfesa Pharma Corporation. YH, ST, HM, and TT are currently employed by Sanofi K.K. HU, YU, AY, SF, YO, HA, KN, and KS declare that they have no conflicts of interest.
Figures
Similar articles
-
Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura.N Engl J Med. 2019 Jan 24;380(4):335-346. doi: 10.1056/NEJMoa1806311. Epub 2019 Jan 9. N Engl J Med. 2019. PMID: 30625070 Clinical Trial.
-
Caplacizumab: a change in the paradigm of thrombotic thrombocytopenic purpura treatment.Expert Opin Biol Ther. 2019 Nov;19(11):1127-1134. doi: 10.1080/14712598.2019.1650908. Epub 2019 Aug 5. Expert Opin Biol Ther. 2019. PMID: 31359806 Review.
-
Persistent ADAMTS13 inhibitor delays recovery of ADAMTS13 activity in caplacizumab-treated Japanese patients with iTTP.Blood Adv. 2024 May 14;8(9):2151-2159. doi: 10.1182/bloodadvances.2023012451. Blood Adv. 2024. PMID: 38386976 Free PMC article.
-
Management of immune thrombotic thrombocytopenic purpura without therapeutic plasma exchange.Blood. 2024 Oct 3;144(14):1486-1495. doi: 10.1182/blood.2023023780. Blood. 2024. PMID: 38838300
-
Refining the standard of care in immune thrombotic thrombocytopenic purpura.Clin Adv Hematol Oncol. 2024 Oct;22(8):381-391. Clin Adv Hematol Oncol. 2024. PMID: 39356816 Review.
Cited by
-
Outcomes and Costs in Patients with Immune Thrombotic Thrombocytopenic Purpura Receiving Front-Line Versus Delayed Caplacizumab: A US Hospital Database Study.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241241525. doi: 10.1177/10760296241241525. Clin Appl Thromb Hemost. 2024. PMID: 38523315 Free PMC article.
-
Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years.J Nanobiotechnology. 2024 Oct 26;22(1):661. doi: 10.1186/s12951-024-02900-y. J Nanobiotechnology. 2024. PMID: 39455963 Free PMC article. Review.
-
Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) in Japan 2023.Int J Hematol. 2023 Nov;118(5):529-546. doi: 10.1007/s12185-023-03657-0. Epub 2023 Sep 10. Int J Hematol. 2023. PMID: 37689812 Free PMC article.
-
The Phenomenon of Thrombotic Microangiopathy in Cancer Patients.Int J Mol Sci. 2024 Aug 21;25(16):9055. doi: 10.3390/ijms25169055. Int J Mol Sci. 2024. PMID: 39201740 Free PMC article. Review.
-
How We Interpret Thrombosis with Thrombocytopenia Syndrome?Int J Mol Sci. 2024 May 1;25(9):4956. doi: 10.3390/ijms25094956. Int J Mol Sci. 2024. PMID: 38732176 Free PMC article. Review.
References
-
- Kayashima M, Sakai K, Harada K, Kanetake J, Kubo M, Hamada E, et al. Strong association between insufficient plasma exchange and fatal outcomes in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura. Int J Hematol. 2021;114:415–423. doi: 10.1007/s12185-021-03197-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

