Tolerogenic nanoparticles induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis
- PMID: 36427299
- PMCID: PMC9699678
- DOI: 10.1126/sciadv.abo5284
Tolerogenic nanoparticles induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis
Abstract
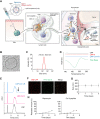
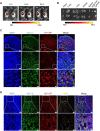
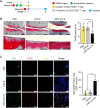
Local inflammation in the joint is considered to contribute to osteoarthritis (OA) progression. Here, we describe an immunomodulating nanoparticle for OA treatment. Intradermal injection of lipid nanoparticles (LNPs) loaded with type II collagen (Col II) and rapamycin (LNP-Col II-R) into OA mice effectively induced Col II-specific anti-inflammatory regulatory T cells, substantially increased anti-inflammatory cytokine expression, and reduced inflammatory immune cells and proinflammatory cytokine expression in the joints. Consequently, LNP-Col II-R injection inhibited chondrocyte apoptosis and cartilage matrix degradation and relieved pain, while injection of LNPs loaded with a control peptide and rapamycin did not induce these events. Adoptive transfer of CD4+CD25+ T cells isolated from LNP-Col II-R-injected mice suggested that Tregs induced by LNP-Col II-R injection were likely responsible for the therapeutic effects. Collectively, this study suggests nanoparticle-mediated immunomodulation in the joint as a simple and effective treatment for OA.
Figures




Comment in
-
Treg cell-inducing nanoparticles show promise for treating OA.Nat Rev Rheumatol. 2023 Feb;19(2):62. doi: 10.1038/s41584-023-00906-8. Nat Rev Rheumatol. 2023. PMID: 36624262 No abstract available.
References
-
- De Lucia O., Murgo A., Pregnolato F., Pontikaki I., De Souza M., Sinelli A., Cimaz R., Caporali R., Hyaluronic acid injections in the treatment of osteoarthritis secondary to primary inflammatory rheumatic diseases: A systematic review and qualitative synthesis. Adv. Ther. 37, 1347–1359 (2020). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

