CRISPR/Cas9-mediated disruption of lipocalins, Ly6g5b, and Ly6g5c causes male subfertility in mice
- PMID: 36428102
- PMCID: PMC10506895
- DOI: 10.1111/andr.13350
CRISPR/Cas9-mediated disruption of lipocalins, Ly6g5b, and Ly6g5c causes male subfertility in mice
Abstract
Background: Spermatozoa become mature and competent for fertilization during transit from the caput epididymis to the cauda epididymis. However, detailed molecular mechanisms of epididymal sperm maturation are still unclear. Here, we focused on multiple epididymis-enriched genes: lipocalin family genes (Lcn5, Lcn6, Lcn8, Lcn9, and Lcn10) and Ly6 family genes (Ly6g5b and Ly6g5c). These genes are evolutionarily conserved in mammals and form clusters on chromosomes 2 and 17 in the mouse, respectively.
Objective: To clarify whether these genes are required for epididymal sperm maturation and acquisition of fertilizing ability, we generated knockout (KO) mice using the CRISPR/Cas9 system and analyzed their phenotype.
Materials and methods: We generated four lines of KO mice: Lcn9 single KO, the lipocalin family quadruple KO (Lcn5, Lcn6, Lcn8, and Lcn10), quintuple KO (Lcn5, Lcn6, Lcn8, Lcn10, and Lcn9), and double KO of Ly6 family genes (Ly6g5b and Ly6g5c).
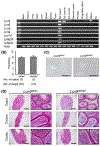
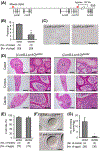
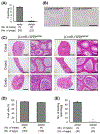
Results: Although the Lcn9 single KO did not affect male fertility, the quadruple KO and quintuple KO male mice were subfertile and mostly infertile, respectively, with a reduced amount of ADAM3, an essential protein for sperm binding to the zona pellucida. Further analysis revealed that the quintuple KO spermatozoa lack the CMTM2A/B that are required for ADAM3 maturation. Intriguingly, Ly6g5b and Ly6g5c double KO male mice also showed subfertility with reduced sperm ADAM3.
Conclusion: These results suggest epididymal secretory proteins are involved in ADAM3 maturation and acquisition of sperm fertilizing ability.
Keywords: Adam3; epididymis; knockout; sperm maturation; zona pellucida.
© 2022 American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


Similar articles
-
Deficiency for Lcn8 causes epididymal sperm maturation defects in mice.Biochem Biophys Res Commun. 2021 Apr 9;548:7-13. doi: 10.1016/j.bbrc.2021.02.052. Epub 2021 Feb 22. Biochem Biophys Res Commun. 2021. PMID: 33631677
-
Multiple genes in the Pate5-13 genomic region contribute to ADAM3 processing†.Biol Reprod. 2024 Apr 11;110(4):750-760. doi: 10.1093/biolre/ioae008. Biol Reprod. 2024. PMID: 38217862 Free PMC article.
-
Nine genes abundantly expressed in the epididymis are not essential for male fecundity in mice.Andrology. 2019 Sep;7(5):644-653. doi: 10.1111/andr.12621. Epub 2019 Mar 29. Andrology. 2019. PMID: 30927342 Free PMC article.
-
Epididymis-specific lipocalin promoters.Asian J Androl. 2007 Jul;9(4):515-21. doi: 10.1111/j.1745-7262.2007.00300.x. Asian J Androl. 2007. PMID: 17589789 Review.
-
Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility.Andrology. 2019 Sep;7(5):610-617. doi: 10.1111/andr.12638. Epub 2019 Jun 19. Andrology. 2019. PMID: 31218833 Review.
Cited by
-
Lumicrine signaling: Extracellular regulation of sperm maturation in the male reproductive tract lumen.Genes Cells. 2023 Nov;28(11):757-763. doi: 10.1111/gtc.13066. Epub 2023 Sep 11. Genes Cells. 2023. PMID: 37696504 Free PMC article. Review.
-
Unlocking Genetic Mysteries during the Epic Sperm Journey toward Fertilization: Further Expanding Cre Mouse Lines.Biomolecules. 2024 Apr 28;14(5):529. doi: 10.3390/biom14050529. Biomolecules. 2024. PMID: 38785936 Free PMC article. Review.
-
Distinct actions of testicular endocrine and lumicrine signaling on the proximal epididymal transcriptome.Reprod Biol Endocrinol. 2024 Apr 10;22(1):40. doi: 10.1186/s12958-024-01213-x. Reprod Biol Endocrinol. 2024. PMID: 38600586 Free PMC article.
-
CUB domains are not required for OVCH2 function in sperm maturation in the mouse epididymis.Andrology. 2024 Mar;12(3):682-697. doi: 10.1111/andr.13508. Epub 2023 Aug 8. Andrology. 2024. PMID: 37551853 Free PMC article.
-
Expression Patterns and Functional Implications of CMTM2 in Human Testis and Sperm.Reprod Sci. 2025 Jul;32(7):2388-2396. doi: 10.1007/s43032-025-01852-4. Epub 2025 Apr 1. Reprod Sci. 2025. PMID: 40167928
References
-
- Hinton BT, Galdamez MM, Sutherland A, et al. How do you get six meters of epididymis inside a human scrotum? Journal of andrology. Nov-Dec 2011;32(6):558–64. - PubMed
-
- Bedford JM. The bearing of epididymal function in strategies for in vitro fertilization and gamete intrafallopian transfer. Ann N Y Acad Sci. 1988;541:284–91. - PubMed
MeSH terms
Substances
Grants and funding
- Senri Life Science Foundation
- P01 HD087157/HD/NICHD NIH HHS/United States
- 22-A-3/National Cerebral and Cardiovascular Center
- JP20KK0155/Japan Society for the Promotion of Science
- Takeda Science Foundation
- R01 HD088412/HD/NICHD NIH HHS/United States
- Mochida Memorial Foundation for Medical and Pharmaceutical Research
- 21-2-6/National Cerebral and Cardiovascular Center
- JP19J00865/Japan Society for the Promotion of Science
- JP21H05033/Japan Society for the Promotion of Science
- Sumitomo Foundation
- JP21K19198/Japan Society for the Promotion of Science
- P01HD087157/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- JP19H05750/Japan Society for the Promotion of Science
- JP21H02397/Japan Society for the Promotion of Science
- 31-6-3/National Cerebral and Cardiovascular Center
- JP21gm5010001/Japan Agency for Medical Research and Development
- INV-001902/GATES/Bill & Melinda Gates Foundation/United States
- R01HD088412/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- 30-2-5/National Cerebral and Cardiovascular Center
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

