Rhizopine biosensors for plant-dependent control of bacterial gene expression
- PMID: 36428208
- PMCID: PMC10107442
- DOI: 10.1111/1462-2920.16288
Rhizopine biosensors for plant-dependent control of bacterial gene expression
Abstract
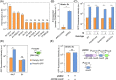
Engineering signalling between plants and microbes could be exploited to establish host-specificity between plant-growth-promoting bacteria and target crops in the environment. We previously engineered rhizopine-signalling circuitry facilitating exclusive signalling between rhizopine-producing (RhiP) plants and model bacterial strains. Here, we conduct an in-depth analysis of rhizopine-inducible expression in bacteria. We characterize two rhizopine-inducible promoters and explore the bacterial host-range of rhizopine biosensor plasmids. By tuning the expression of rhizopine uptake genes, we also construct a new biosensor plasmid pSIR05 that has minimal impact on host cell growth in vitro and exhibits markedly improved stability of expression in situ on RhiP barley roots compared to the previously described biosensor plasmid pSIR02. We demonstrate that a sub-population of Azorhizobium caulinodans cells carrying pSIR05 can sense rhizopine and activate gene expression when colonizing RhiP barley roots. However, these bacteria were mildly defective for colonization of RhiP barley roots compared to the wild-type parent strain. This work provides advancement towards establishing more robust plant-dependent control of bacterial gene expression and highlights the key challenges remaining to achieve this goal.
© 2022 The Authors. Environmental Microbiology published by Applied Microbiology International and John Wiley & Sons Ltd.
Conflict of interest statement
The authors disclose no conflicts of interest relating to this work.
Figures




Similar articles
-
Engineered plant control of associative nitrogen fixation.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2117465119. doi: 10.1073/pnas.2117465119. Epub 2022 Apr 11. Proc Natl Acad Sci U S A. 2022. PMID: 35412890 Free PMC article.
-
Control of nitrogen fixation and ammonia excretion in Azorhizobium caulinodans.PLoS Genet. 2022 Jun 21;18(6):e1010276. doi: 10.1371/journal.pgen.1010276. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35727841 Free PMC article.
-
Engineering transkingdom signalling in plants to control gene expression in rhizosphere bacteria.Nat Commun. 2019 Jul 31;10(1):3430. doi: 10.1038/s41467-019-10882-x. Nat Commun. 2019. PMID: 31366919 Free PMC article.
-
Strategies for Improving Small-Molecule Biosensors in Bacteria.Biosensors (Basel). 2022 Jan 25;12(2):64. doi: 10.3390/bios12020064. Biosensors (Basel). 2022. PMID: 35200325 Free PMC article. Review.
-
Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants.Microbiol Res. 2019 Apr;221:36-49. doi: 10.1016/j.micres.2019.02.001. Epub 2019 Feb 4. Microbiol Res. 2019. PMID: 30825940 Review.
Cited by
-
Regulation of Rhizobial Nodulation Genes by Flavonoid-Independent NodD Supports Nitrogen-Fixing Symbioses With Legumes.Environ Microbiol. 2025 Jan;27(1):e70014. doi: 10.1111/1462-2920.70014. Environ Microbiol. 2025. PMID: 39865396 Free PMC article.
-
Scripting a new dialogue between diazotrophs and crops.Trends Microbiol. 2024 Jun;32(6):577-589. doi: 10.1016/j.tim.2023.08.007. Epub 2023 Sep 26. Trends Microbiol. 2024. PMID: 37770375 Free PMC article. Review.
References
-
- Bahar, M. , Majnik, D. , Wexler, M.M.W. , Fry, J. , Poole, P.S. & Murphy, P.J. (1998) A model for the catabolism of rhizopine in Rhizobium leguminosarum involves a ferredoxin oxygenase complex and the inositol degradative pathway. Molecular Plant‐Microbe Interactions, 11(11), 1057–1068. - PubMed
-
- Bai, B. , Liu, W. , Qiu, X. , Zhang, J. , Zhang, J. & Bai, Y. (2022) The root microbiome: community assembly and its contributions to plant fitness. Journal of Integrative Plant Biology, 64, 230–243. - PubMed
-
- Belitsky, B.R. (2004) Bacillus subtilis GabR, a protein with DNA‐binding and aminotransferase domains, is a PLP‐dependent transcriptional regulator. Journal of Molecular Biology, 340, 655–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

