Brain Organoids to Evaluate Cellular Therapies
- PMID: 36428378
- PMCID: PMC9686900
- DOI: 10.3390/ani12223150
Brain Organoids to Evaluate Cellular Therapies
Abstract
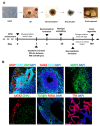
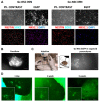
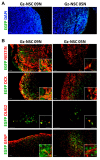
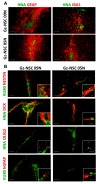
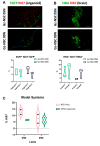
Animal models currently used to test the efficacy and safety of cell therapies, mainly murine models, have limitations as molecular, cellular, and physiological mechanisms are often inherently different between species, especially in the brain. Therefore, for clinical translation of cell-based medicinal products, the development of alternative models based on human neural cells may be crucial. We have developed an in vitro model of transplantation into human brain organoids to study the potential of neural stem cells as cell therapeutics and compared these data with standard xenograft studies in the brain of immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. Neural stem cells showed similar differentiation and proliferation potentials in both human brain organoids and mouse brains. Our results suggest that brain organoids can be informative in the evaluation of cell therapies, helping to reduce the number of animals used for regulatory studies.
Keywords: 3 Rs; brain organoids; cell therapy; neural progenitors; neural stem cells; reduction; translation.
Conflict of interest statement
J.M.-R., R.S.-P. and B.F.-M are authors of a patent application for the use of Gz-NSC (nº application European Patent Office: 200930943). The other authors indicate no potential conflict of interest.
Figures
References
-
- Donega V., Nijboer C.H., Van Velthoven C.T.J., Youssef S.A., De Bruin A., Van Bel F., Kavelaars A., Heijnen C.J. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr. Res. 2015;78:520–526. doi: 10.1038/pr.2015.145. - DOI - PMC - PubMed

