mTOR Signaling in BDNF-Treated Guinea Pigs after Ototoxic Deafening
- PMID: 36428503
- PMCID: PMC9687683
- DOI: 10.3390/biomedicines10112935
mTOR Signaling in BDNF-Treated Guinea Pigs after Ototoxic Deafening
Abstract
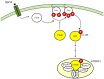
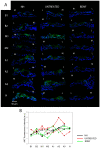
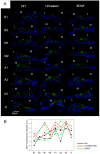
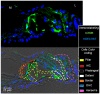
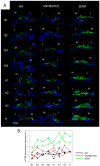
The mammalian target of rapamycin (mTOR) signaling plays a critical role in cell homeostasis, growth and survival. Here, we investigated the localization of the main mTOR signaling proteins in the organ of Corti of normal-hearing and deafened guinea pigs, as well as their possible modulation by exogenously administered brain-derived neurotrophic factor (BDNF) in deafened guinea pigs. Animals were ototoxically deafened by systemic administration of kanamycin and furosemide, and one week later, the right cochleas were treated with gelatin sponge soaked in rhBDNF, while the left cochleas were used as negative controls. Twenty-four hours after treatment, animals were euthanized, and the cochleas were processed for subsequent analysis. Through immunofluorescence, we demonstrated the localization of AKT, pAKT, mTOR, pmTOR and PTEN proteins throughout the cochlea of guinea pigs for the first time, with a higher expression in supporting cells. Moreover, an increase in mTOR immunostaining was observed in BDNF-treated cochleas by means of fluorescence intensity compared to the other groups. Conversely, Western blot analysis showed no significant differences in the protein levels between groups, probably due to dilution of proteins in the neighboring tissues of the organ of Corti. Altogether, our data indicate that mTOR signaling proteins are expressed by the organ of Corti (with a major role for supporting cells) and that the modulation of mTOR may be a protective mechanism triggered by BDNF in the degenerating organ of Corti.
Keywords: BDNF; Western blot; hair cells; hearing loss; immunofluorescence; mTOR; neurotrophins; organ of Corti; supporting cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Ramekers D., Versnel H., Grolman W., Klis S.F.L. Neurotrophins and their role in the cochlea. Hear. Res. 2012;288:19–33. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

