Modeling Patient-Specific CAR-T Cell Dynamics: Multiphasic Kinetics via Phenotypic Differentiation
- PMID: 36428671
- PMCID: PMC9688514
- DOI: 10.3390/cancers14225576
Modeling Patient-Specific CAR-T Cell Dynamics: Multiphasic Kinetics via Phenotypic Differentiation
Abstract
Chimeric Antigen Receptor (CAR)-T cell immunotherapy revolutionized cancer treatment and consists of the genetic modification of T lymphocytes with a CAR gene, aiming to increase their ability to recognize and kill antigen-specific tumor cells. The dynamics of CAR-T cell responses in patients present multiphasic kinetics with distribution, expansion, contraction, and persistence phases. The characteristics and duration of each phase depend on the tumor type, the infused product, and patient-specific characteristics. We present a mathematical model that describes the multiphasic CAR-T cell dynamics resulting from the interplay between CAR-T and tumor cells, considering patient and product heterogeneities. The CAR-T cell population is divided into functional (distributed and effector), memory, and exhausted CAR-T cell phenotypes. The model is able to describe the diversity of CAR-T cell dynamical behaviors in different patients and hematological cancers as well as their therapy outcomes. Our results indicate that the joint assessment of the area under the concentration-time curve in the first 28 days and the corresponding fraction of non-exhausted CAR-T cells may be considered a potential marker to classify therapy responses. Overall, the analysis of different CAR-T cell phenotypes can be a key aspect for a better understanding of the whole CAR-T cell dynamics.
Keywords: CAR-T cell exhaustion; antigen dependent CAR-T expansion; functional CAR-T cells; hematological malignancies; memory pool; treatment outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


 ) of CAR-T cell kinetics from a representative patient profile were split among the four phases of CAR-T cell dynamics to which lines in the logplot of the CAR-T cell population along time were fitted. The corresponding (growth or decline) rates are denoted by , and , associated with the distribution, expansion, contraction, and persistence phases, respectively. These rates are used as first approximations to the parameters of the leading mechanism(s) of each phase. Specifically, the distribution phase is mainly driven by the reduction rate of the injected CAR-T cells so that ; the expansion phase is driven by the combined effect between the expansion () and mortality (), leading to ; the contraction and persistence phases are mainly driven by the mortality of exhausted and memory CAR-T cells, respectively, which yield and . (b) The per capita rate of the total CAR-T cell population () is displayed over time after infusion together with the calibrated values of , , , and .
) of CAR-T cell kinetics from a representative patient profile were split among the four phases of CAR-T cell dynamics to which lines in the logplot of the CAR-T cell population along time were fitted. The corresponding (growth or decline) rates are denoted by , and , associated with the distribution, expansion, contraction, and persistence phases, respectively. These rates are used as first approximations to the parameters of the leading mechanism(s) of each phase. Specifically, the distribution phase is mainly driven by the reduction rate of the injected CAR-T cells so that ; the expansion phase is driven by the combined effect between the expansion () and mortality (), leading to ; the contraction and persistence phases are mainly driven by the mortality of exhausted and memory CAR-T cells, respectively, which yield and . (b) The per capita rate of the total CAR-T cell population () is displayed over time after infusion together with the calibrated values of , , , and .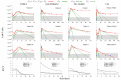
 ) from [22]. Each column corresponds to the dynamics of the total CAR-T cell population (
) from [22]. Each column corresponds to the dynamics of the total CAR-T cell population ( ) for different diseases (DLBCL, pediatric and adult ALL, and CLL) and different patients. The total CAR-T cell population is divided into effector (), memory (), and exhausted () phenotypes, shown in continuous, dashed, and dotted green, respectively. The mean dose value of cells (
) for different diseases (DLBCL, pediatric and adult ALL, and CLL) and different patients. The total CAR-T cell population is divided into effector (), memory (), and exhausted () phenotypes, shown in continuous, dashed, and dotted green, respectively. The mean dose value of cells ( ) presented in [21] is used as a surrogate for the actual doses when not reported for patients with ALL. The gray region represents the undetectable levels (below the threshold of cells to DLBCL and pediatric ALL, cells to adult ALL, and cells to CLL [22]). Data points in this region (
) presented in [21] is used as a surrogate for the actual doses when not reported for patients with ALL. The gray region represents the undetectable levels (below the threshold of cells to DLBCL and pediatric ALL, cells to adult ALL, and cells to CLL [22]). Data points in this region ( ) were not used for calibration and error calculation due to their high uncertainties. The bottom row presents the time-dependent expansion rate function () for each patient.
) were not used for calibration and error calculation due to their high uncertainties. The bottom row presents the time-dependent expansion rate function () for each patient.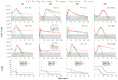
 ) from [37,38]. Each column corresponds to the dynamics of the total CAR-T cell population (
) from [37,38]. Each column corresponds to the dynamics of the total CAR-T cell population ( ) for different therapy responses at the last follow-up (interval from infusion to the last follow-up in days) (CR—complete response, PR—partial response, and SD—stable disease) and different patients. The total CAR-T cell population is divided into effector (), memory (), and exhausted () phenotypes, shown in continuous, dashed, and dotted green, respectively. The gray region represents the undetectable level. Data points (
) for different therapy responses at the last follow-up (interval from infusion to the last follow-up in days) (CR—complete response, PR—partial response, and SD—stable disease) and different patients. The total CAR-T cell population is divided into effector (), memory (), and exhausted () phenotypes, shown in continuous, dashed, and dotted green, respectively. The gray region represents the undetectable level. Data points ( ) may assume any value in this region, but some (
) may assume any value in this region, but some ( ) were not used for calibration and error calculation of the model due to their greater uncertainty. The bottom row presents the time-dependent expansion rate function () for each patient.
) were not used for calibration and error calculation of the model due to their greater uncertainty. The bottom row presents the time-dependent expansion rate function () for each patient.

References
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

