The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment
- PMID: 36428684
- PMCID: PMC9688166
- DOI: 10.3390/cancers14225594
The Expression of Cell Cycle-Related Genes in USP8-Mutated Corticotroph Neuroendocrine Pituitary Tumors and Their Possible Role in Cell Cycle-Targeting Treatment
Abstract
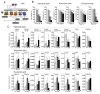
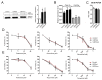
Protein deubiquitinases USP8 and USP48 are known driver genes in corticotroph pituitary neuroendocrine tumors (PitNETs). USP8 mutations have pleiotropic effects that include notable changes in genes' expression. Genes involved in cell cycle regulation were found differentially expressed in mutated and wild-type tumors. This study aimed to verify difference in the expression level of selected cell cycle-related genes and investigate their potential role in response to cell cycle inhibitors. Analysis of 70 corticotroph PitNETs showed that USP8-mutated tumors have lower CDKN1B, CDK6, CCND2 and higher CDC25A expression. USP48-mutated tumors have lower CDKN1B and CCND1 expression. A lower p27 protein level in mutated than in wild-type tumors was confirmed that may potentially influence the response to small molecule inhibitors targeting the cell cycle. We looked for the role of USP8 mutations or a changed p27 level in the response to palbociclib, flavopiridol and roscovitine in vitro using murine corticotroph AtT-20/D16v-F2 cells. The cells were sensitive to each agent and treatment influenced the expression of genes involved in cell cycle regulation. Overexpression of mutated Usp8 in the cells did not affect the expression of p27 nor the response to the inhibitors. Downregulating or upregulating p27 expression in AtT-20/D16v-F2 cells also did not affect treatment response.
Keywords: Cushing’s disease; USP48; USP8; cell cycle; corticotroph PitNET; flavopiridol; p27; palbociclib; pituitary; roscovitine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bujko M., Kober P., Boresowicz J., Rusetska N., Paziewska A., Daąbrowska M., Piaścik A., Pȩkul M., Zieliński G., Kunicki J., et al. USP8 Mutations in Corticotroph Adenomas Determine a Distinct Gene Expression Profile Irrespective of Functional Tumour Status. Eur. J. Endocrinol. 2019;181:615–627. doi: 10.1530/EJE-19-0194. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

