The NAMPT Inhibitor FK866 Increases Metformin Sensitivity in Pancreatic Cancer Cells
- PMID: 36428689
- PMCID: PMC9688551
- DOI: 10.3390/cancers14225597
The NAMPT Inhibitor FK866 Increases Metformin Sensitivity in Pancreatic Cancer Cells
Abstract
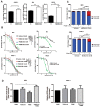
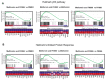
Pancreatic cancer (pancreatic ductal adenocarcinoma: PDAC) is one of the most aggressive neoplastic diseases. Metformin use has been associated with reduced pancreatic cancer incidence and better survival in diabetics. Metformin has been shown to inhibit PDAC cells growth and survival, both in vitro and in vivo. However, clinical trials using metformin have failed to reduce pancreatic cancer progression in patients, raising important questions about molecular mechanisms that protect tumor cells from the antineoplastic activities of metformin. We confirmed that metformin acts through inhibition of mitochondrial complex I, decreasing the NAD+/NADH ratio, and that NAD+/NADH homeostasis determines metformin sensitivity in several cancer cell lines. Metabolites that can restore the NAD+/NADH ratio caused PDAC cells to be resistant to metformin. In addition, metformin treatment of PDAC cell lines induced a compensatory NAMPT expression, increasing the pool of cellular NAD+. The NAMPT inhibitor FK866 sensitized PDAC cells to the antiproliferative effects of metformin in vitro and decreased the cellular NAD+ pool. Intriguingly, FK866 combined with metformin increased survival in mice bearing KP4 cell line xenografts, but not in mice with PANC-1 cell line xenografts. Transcriptome analysis revealed that the drug combination reactivated genes in the p53 pathway and oxidative stress, providing new insights about the mechanisms leading to cancer cell death.
Keywords: NAD; NAMPT; metabolism; metformin; pancreatic cancer.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Reni M., Dugnani E., Cereda S., Belli C., Balzano G., Nicoletti R., Liberati D., Pasquale V., Scavini M., Maggiora P., et al. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin. Cancer Res. 2016;22:1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

