2-Deoxyglucose, an Inhibitor of Glycolysis, Enhances the Oncolytic Effect of Coxsackievirus
- PMID: 36428704
- PMCID: PMC9688421
- DOI: 10.3390/cancers14225611
2-Deoxyglucose, an Inhibitor of Glycolysis, Enhances the Oncolytic Effect of Coxsackievirus
Abstract
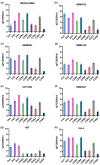
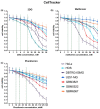
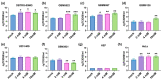
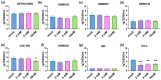
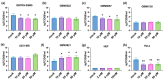
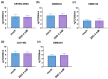
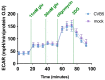
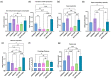
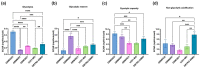
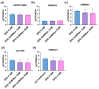
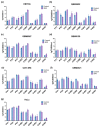
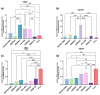
Glioblastoma multiforme (GBM) is one of the most common types of brain tumor. Despite intensive research, patients with GBM have a poor prognosis due to a very high rate of relapse and significant side effects of the treatment, with a median survival of 14.6 months. Oncolytic viruses are considered a promising strategy to eliminate GBM and other types of cancer, and several viruses have already been introduced into clinical practice. However, identification of the factors that underly the sensitivity of tumor species to oncolytic viruses or that modulate their clinical efficacy remains an important target. Here, we show that Coxsackievirus B5 (CVB5) demonstrates high oncolytic potential towards GBM primary cell species and cell lines. Moreover, 2-deoxyglucose (2DG), an inhibitor of glycolysis, potentiates the cytopathic effects of CVB5 in most of the cancer cell lines tested. The cells in which the inhibition of glycolysis enhanced oncolysis are characterized by high mitochondrial respiratory activity and glycolytic capacity, as determined by Seahorse analysis. Thus, 2-deoxyglucose and other analogs should be considered as adjuvants for oncolytic therapy of glioblastoma multiforme.
Keywords: 2-deoxyglucose; coxsackievirus; metabolism; oncolytic virus; seahorse.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Oncolytic viruses as a promising therapeutic strategy against the detrimental health impacts of air pollution: The case of glioblastoma multiforme.Semin Cancer Biol. 2022 Nov;86(Pt 3):1122-1142. doi: 10.1016/j.semcancer.2021.05.013. Epub 2021 May 15. Semin Cancer Biol. 2022. PMID: 34004331 Review.
-
Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin.Neuro Oncol. 2017 Feb 1;19(2):197-207. doi: 10.1093/neuonc/now174. Neuro Oncol. 2017. PMID: 27571886 Free PMC article.
-
CDK4/6 Inhibition Enhances Oncolytic Virus Efficacy by Potentiating Tumor-Selective Cell Killing and T-cell Activation in Refractory Glioblastoma.Cancer Res. 2022 Sep 16;82(18):3359-3374. doi: 10.1158/0008-5472.CAN-21-3656. Cancer Res. 2022. PMID: 35792620
-
Characterization and oncolytic virus targeting of FAP-expressing tumor-associated pericytes in glioblastoma.Acta Neuropathol Commun. 2020 Dec 11;8(1):221. doi: 10.1186/s40478-020-01096-0. Acta Neuropathol Commun. 2020. PMID: 33308315 Free PMC article.
-
Virus-Based Immunotherapy of Glioblastoma.Cancers (Basel). 2019 Feb 5;11(2):186. doi: 10.3390/cancers11020186. Cancers (Basel). 2019. PMID: 30764570 Free PMC article. Review.
Cited by
-
Transcriptome Analysis of Redox Systems and Polyamine Metabolic Pathway in Hepatoma and Non-Tumor Hepatocyte-like Cells.Biomolecules. 2023 Apr 21;13(4):714. doi: 10.3390/biom13040714. Biomolecules. 2023. PMID: 37189460 Free PMC article.
-
Improved Natamycin Production in Streptomyces gilvosporeus Through Mutagenesis and Enhanced Nitrogen Metabolism.Microorganisms. 2025 Feb 10;13(2):390. doi: 10.3390/microorganisms13020390. Microorganisms. 2025. PMID: 40005756 Free PMC article.
-
Targeting Glucose Metabolism in Cancer Cells as an Approach to Overcoming Drug Resistance.Pharmaceutics. 2023 Nov 10;15(11):2610. doi: 10.3390/pharmaceutics15112610. Pharmaceutics. 2023. PMID: 38004589 Free PMC article. Review.
-
An Update on the Clinical Status, Challenges, and Future Directions of Oncolytic Virotherapy for Malignant Gliomas.Curr Treat Options Oncol. 2024 Jul;25(7):952-991. doi: 10.1007/s11864-024-01211-6. Epub 2024 Jun 19. Curr Treat Options Oncol. 2024. PMID: 38896326 Free PMC article. Review.
-
Generating Shigella that internalize into glioblastoma cells.Front Oncol. 2023 Nov 23;13:1229747. doi: 10.3389/fonc.2023.1229747. eCollection 2023. Front Oncol. 2023. PMID: 38074687 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

