Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges
- PMID: 36428722
- PMCID: PMC9688380
- DOI: 10.3390/cancers14225630
Cancer Patients and the COVID-19 Vaccines: Considerations and Challenges
Abstract
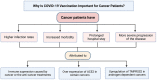
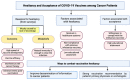
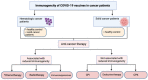
Few guidelines exist for COVID-19 vaccination amongst cancer patients, fostering uncertainty regarding the immunogenicity, safety, and effects of cancer therapies on vaccination, which this review aims to address. A literature review was conducted to include the latest articles covering the immunogenicity and safety of COVID-19 vaccination in patients with solid and hematologic cancers receiving various treatments. Lower seropositivity following vaccination was associated with malignancy (compared to the general population), and hematologic malignancy (compared to solid cancers). Patients receiving active cancer therapy (unspecified), chemotherapy, radiotherapy, and immunosuppressants generally demonstrated lower seropositivity compared to healthy controls; though checkpoint inhibition, endocrine therapy, and cyclin dependent kinase inhibition did not appear to affect seropositivity. Vaccination appeared safe and well-tolerated in patients with current or past cancer and those undergoing treatment. Adverse events were comparable to the general population, but inflammatory lymphadenopathy following vaccination was commonly reported and may be mistaken for malignant etiology. Additionally, radiation recall phenomenon was sporadically reported in patients who had received radiotherapy. Overall, while seropositivity rates were decreased, cancer patients showed capacity to generate safe and effective immune responses to COVID-19 vaccination, thus vaccination should be encouraged and hesitancy should be addressed in this population.
Keywords: COVID-19; cancer; cancer therapies; immunogenicity; safety; vaccination; vaccine hesitancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
COVID-19 and Cancer: Special Considerations for Patients Receiving Immunotherapy and Immunosuppressive Cancer Therapies.Am Soc Clin Oncol Educ Book. 2022 Apr;42:1-13. doi: 10.1200/EDBK_359656. Am Soc Clin Oncol Educ Book. 2022. PMID: 35658503
-
Safety and immunogenicity of the COVID-19 vaccine BNT162b2 in patients undergoing chemotherapy for solid cancer.J Infect Chemother. 2022 Apr;28(4):516-520. doi: 10.1016/j.jiac.2021.12.021. Epub 2021 Dec 30. J Infect Chemother. 2022. PMID: 35090826 Free PMC article.
-
Immunogenicity of COVID-19 vaccines in patients with diverse health conditions: A comprehensive systematic review.J Med Virol. 2022 Sep;94(9):4144-4155. doi: 10.1002/jmv.27828. Epub 2022 May 27. J Med Virol. 2022. PMID: 35567325 Free PMC article.
-
Immunogenicity and Safety of COVID-19 Vaccines in Patients Receiving Renal Replacement Therapy: A Systematic Review and Meta-Analysis.Front Med (Lausanne). 2022 Mar 9;9:827859. doi: 10.3389/fmed.2022.827859. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35355604 Free PMC article.
-
COVID-19 vaccination rates, intent, and hesitancy in patients with solid organ and blood cancers: A multicenter study.Asia Pac J Clin Oncol. 2022 Dec;18(6):570-577. doi: 10.1111/ajco.13754. Epub 2022 Jan 18. Asia Pac J Clin Oncol. 2022. PMID: 35043559
Cited by
-
COVID-19 Vaccination in Patients With Cancer and Patients Receiving HSCT or CAR-T Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future.J Infect Dis. 2023 Aug 4;228(Suppl 1):S55-S69. doi: 10.1093/infdis/jiad174. J Infect Dis. 2023. PMID: 37539765 Free PMC article. Review.
-
Insights into the Impact of Hesitancy on Cancer Care and COVID-19.Cancers (Basel). 2023 Jun 8;15(12):3115. doi: 10.3390/cancers15123115. Cancers (Basel). 2023. PMID: 37370725 Free PMC article. Review.
-
Clinical impact of concurrent autologous adoptive T cells immunotherapy in active COVID-19 infected cancer patients for chemotherapy.Infect Agent Cancer. 2025 Apr 9;20(1):23. doi: 10.1186/s13027-025-00654-2. Infect Agent Cancer. 2025. PMID: 40205403 Free PMC article.
-
COVID-19 vaccination patterns among oral cancer patients: A comprehensive analysis in a medical center in Taiwan.J Dent Sci. 2025 Jan;20(1):335-341. doi: 10.1016/j.jds.2024.06.020. Epub 2024 Jul 12. J Dent Sci. 2025. PMID: 39873075 Free PMC article.
-
Perspectives and Challenges of COVID-19 with Obesity-Related Cancers.Cancers (Basel). 2023 Mar 15;15(6):1771. doi: 10.3390/cancers15061771. Cancers (Basel). 2023. PMID: 36980657 Free PMC article.
References
-
- Yasin A.I., Aydin S.G., Sümbül B., Koral L., Simşek M., Geredeli Ç., Öztürk A., Perkin P., Demirtaş D., Erdemoglu E., et al. Efficacy and Safety Profile of COVID-19 Vaccine in Cancer Patients: A Prospective, Multicenter Cohort Study. Futur. Oncol. 2022;18:1235–1244. doi: 10.2217/fon-2021-1248. - DOI - PMC - PubMed
-
- Matovina Brko G., Popovic M., Jovic M., Radic J., Bodlovic Kladar M., Nikolic I., Vidovic V., Kolarov Bjelobrk I., Kukic B., Salma S., et al. COVID-19 Vaccines and Cancer Patients: Acceptance, Attitudes and Safety. JBUON. 2021;26:2188–2195. - PubMed
-
- Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and Immunogenicity of One versus Two Doses of the COVID-19 Vaccine BNT162b2 for Patients with Cancer: Interim Analysis of a Prospective Observational Study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

