Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1
- PMID: 36428739
- PMCID: PMC9688283
- DOI: 10.3390/cancers14225648
Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1
Abstract
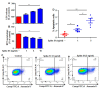
This study underlines the importance of SARS-CoV-2 spike S1 in prompting death in cultured non-small cell lung cancer (NSCLC) cells and in vivo in lung tumors in mice. Interestingly, we found that recombinant spike S1 treatment at very low doses led to death of human A549 NSCLC cells. On the other hand, boiled recombinant SARS-CoV-2 spike S1 remained unable to induce death, suggesting that the induction of cell death in A549 cells was due to native SARS-CoV-2 spike S1 protein. SARS-CoV-2 spike S1-induced A549 cell death was also inhibited by neutralizing antibodies against spike S1 and ACE2. Moreover, our newly designed wild type ACE2-interacting domain of SARS-CoV-2 (wtAIDS), but not mAIDS, peptide also attenuated SARS-CoV-2 spike S1-induced cell death, suggesting that SARS-CoV-2 spike S1-induced death in A549 NSCLC cells depends on its interaction with ACE2 receptor. Similarly, recombinant spike S1 treatment also led to death of human H1299 and H358 NSCLC cells. Finally, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) intoxication led to the formation tumors in lungs of A/J mice and alternate day intranasal treatment with low dose of recombinant SARS-CoV-2 spike S1 from 22-weeks of NNK insult (late stage) induced apoptosis and tumor regression in the lungs. These studies indicate that SARS-CoV-2 spike S1 may have implications for lung cancer treatment.
Keywords: ACE2; NNK mouse model of lung cancer; SARS-CoV-2 spike S1; apoptosis; human lung cancer cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Centers for Disease Control Chronic disease reports: Deaths from lung cancer--United States, 1986. JAMA. 1989;262:1170–1172. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

