The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments
- PMID: 36428792
- PMCID: PMC9688539
- DOI: 10.3390/cancers14225700
The Multidisciplinary Approach in Stage III Non-Small Cell Lung Cancer over Ten Years: From Radiation Therapy Optimisation to Innovative Systemic Treatments
Abstract
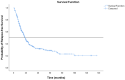
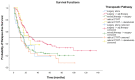
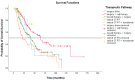
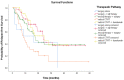
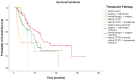
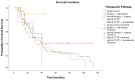
Background: About 30% of new non-small cell lung cancer (NSCLC) cases are diagnosed at a locally advanced stage, which includes a highly heterogeneous group of patients with a wide spectrum of treatment options. The management of stage III NSCLC involves a multidisciplinary team, adequate staging, and a careful patient selection for surgery or radiation therapy integrated with systemic treatment. Methods: This is a single-center observational retrospective and prospective study including a consecutive series of stage III NSCLC patients who were referred to the Veneto Institute of Oncology and University Hospital of Padova (Italy) between 2012 and 2021. We described clinico-pathological characteristics, therapeutic pathways, and treatment responses in terms of radiological response in the entire study population and in terms of pathological response in patients who underwent surgery after induction therapy. Furthermore, we analysed survival outcomes in terms of relapse-free survival (RFS) and overall survival (OS). Results: A total of 301 patients were included. The majority of patients received surgical multimodality treatment (n = 223, 74.1%), while the remaining patients (n = 78, 25.9%) underwent definitive CRT followed or not by durvalumab as consolidation therapy. At data cut-off, 188 patients (62.5%) relapsed and the median RFS (mRFS) of the entire population was 18.2 months (95% CI: 15.83−20.57). At the time of analyses 140 patients (46.5%) were alive and the median OS (mOS) was 44.7 months (95% CI: 38.4−51.0). A statistically significant difference both in mRFS (p = 0.002) and in mOS (p < 0.001) was observed according to the therapeutic pathway in the entire population, and selecting patients treated after 2018, a significant difference in mRFS (p = 0.006) and mOS (p < 0.001) was observed according to treatment modality. Furthermore, considering only patients diagnosed with stage IIIB-C (N = 131, 43.5%), there were significant differences both in mRFS (p = 0.047) and in mOS (p = 0.022) as per the treatment algorithm. The mRFS of the unresectable population was 16.3 months (95% CI: 11.48−21.12), with a significant difference among subgroups (p = 0.030) in favour of patients who underwent the PACIFIC-regimen; while the mOS was 46.5 months (95% CI: 26.46−66.65), with a significant difference between two subgroups (p = 0.003) in favour of consolidation immunotherapy. Conclusions: Our work provides insights into the management and the survival outcomes of stage III NSCLC over about 10 years. We found that the choice of radical treatment impacts on outcome, thus suggesting the importance of appropriate staging at diagnosis, patient selection, and of the multidisciplinary approach in the decision-making process. Our results confirmed that the PACIFIC trial and the following introduction of durvalumab as consolidation treatment may be considered as a turning point for several improvements in the diagnostic-therapeutic pathway of stage III NSCLC patients.
Keywords: PACIFIC regimen; concurrent chemo-radiation therapy; immunotherapy; multidisciplinary team; multimodality treatment; neoadjuvant therapy; radiotherapy; stage III NSCLC.
Conflict of interest statement
The authors declare no conflict of interest regarding the content of this manuscript.
Figures















References
-
- Lung and Bronchus Cancer—Cancer Stat Facts. [(accessed on 1 October 2022)]; Available online: https://seer.cancer.gov/statfacts/html/lungb.html.
-
- Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V., et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

