Lateral Flow Immunoassays for SARS-CoV-2
- PMID: 36428918
- PMCID: PMC9689684
- DOI: 10.3390/diagnostics12112854
Lateral Flow Immunoassays for SARS-CoV-2
Abstract
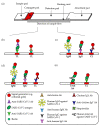
The continued circulation of SARS-CoV-2 virus in different parts of the world opens up the possibility for more virulent variants to evolve even as the coronavirus disease 2019 transitions from pandemic to endemic. Highly transmissible and virulent variants may seed new disruptive epidemic waves that can easily put the healthcare system under tremendous pressure. Despite various nucleic acid-based diagnostic tests that are now commercially available, the wide applications of these tests are largely hampered by specialized equipment requirements that may not be readily available, accessible and affordable in less developed countries or in low resource settings. Hence, the availability of lateral flow immunoassays (LFIs), which can serve as a diagnostic tool by detecting SARS-CoV-2 antigen or as a serological tool by measuring host immune response, is highly appealing. LFI is rapid, low cost, equipment-free, scalable for mass production and ideal for point-of-care settings. In this review, we first summarize the principle and assay format of these LFIs with emphasis on those that were granted emergency use authorization by the US Food and Drug Administration followed by discussion on the specimen type, marker selection and assay performance. We conclude with an overview of challenges and future perspective of LFI applications.
Keywords: antibody; antigen; diagnostic; dipstick; immunochromatography; immunosensor; point-of-care; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
FnCas9-based CRISPR diagnostic for rapid and accurate detection of major SARS-CoV-2 variants on a paper strip.Elife. 2021 Jun 9;10:e67130. doi: 10.7554/eLife.67130. Elife. 2021. PMID: 34106048 Free PMC article.
-
Technical considerations to development of serological tests for SARS-CoV-2.Talanta. 2021 Mar 1;224:121883. doi: 10.1016/j.talanta.2020.121883. Epub 2020 Nov 10. Talanta. 2021. PMID: 33379092 Free PMC article. Review.
-
SARS-CoV-2 Coronavirus Nucleocapsid Antigen-Detecting Half-Strip Lateral Flow Assay Toward the Development of Point of Care Tests Using Commercially Available Reagents.Anal Chem. 2020 Aug 18;92(16):11305-11309. doi: 10.1021/acs.analchem.0c01975. Epub 2020 Aug 5. Anal Chem. 2020. PMID: 32605363
-
COVID-19 in-vitro Diagnostics: State-of-the-Art and Challenges for Rapid, Scalable, and High-Accuracy Screening.Front Bioeng Biotechnol. 2021 Jan 28;8:605702. doi: 10.3389/fbioe.2020.605702. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33634079 Free PMC article. Review.
-
Development and Efficacy of Lateral Flow Point-of-Care Testing Devices for Rapid and Mass COVID-19 Diagnosis by the Detections of SARS-CoV-2 Antigen and Anti-SARS-CoV-2 Antibodies.Diagnostics (Basel). 2021 Sep 24;11(10):1760. doi: 10.3390/diagnostics11101760. Diagnostics (Basel). 2021. PMID: 34679458 Free PMC article. Review.
Cited by
-
Clinical Evaluation of the Healgen Rapid COVID-19 Antigen Test as a Point-of-Care Diagnostic Tool.Immun Inflamm Dis. 2025 Jul;13(7):e70228. doi: 10.1002/iid3.70228. Immun Inflamm Dis. 2025. PMID: 40728074 Free PMC article.
-
Validation of a MALDI-TOF MS Method for SARS-CoV-2 Detection on the Bruker Biotyper and Nasopharyngeal Swabs: A Brazil-UK Collaborative Study.Diagnostics (Basel). 2023 Apr 19;13(8):1470. doi: 10.3390/diagnostics13081470. Diagnostics (Basel). 2023. PMID: 37189571 Free PMC article.
-
Detection of IgG Antibodies Against COVID-19 N-Protein by Hybrid Graphene-Nanorod Sensor.Biosensors (Basel). 2025 Mar 4;15(3):164. doi: 10.3390/bios15030164. Biosensors (Basel). 2025. PMID: 40136961 Free PMC article.
-
Temporal Trend of the SARS-CoV-2 Omicron Variant and RSV in the Nasal Cavity and Accuracy of the Newly Developed Antigen-Detecting Rapid Diagnostic Test.Diagnostics (Basel). 2024 Jan 4;14(1):119. doi: 10.3390/diagnostics14010119. Diagnostics (Basel). 2024. PMID: 38201428 Free PMC article.
-
An update on lateral flow immunoassay for the rapid detection of SARS-CoV-2 antibodies.AIMS Microbiol. 2023 Apr 13;9(2):375-401. doi: 10.3934/microbiol.2023020. eCollection 2023. AIMS Microbiol. 2023. PMID: 37091823 Free PMC article. Review.
References
-
- World Health Organization Weekly Epidemiological Update on COVID-19. [(accessed on 5 October 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. - DOI - PMC - PubMed
-
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

