Actin-Binding Proteins in Cardiac Hypertrophy
- PMID: 36428995
- PMCID: PMC9688942
- DOI: 10.3390/cells11223566
Actin-Binding Proteins in Cardiac Hypertrophy
Abstract
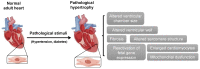
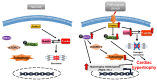
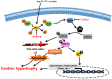
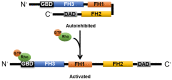
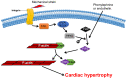
The heart reacts to a large number of pathological stimuli through cardiac hypertrophy, which finally can lead to heart failure. However, the molecular mechanisms of cardiac hypertrophy remain elusive. Actin participates in the formation of highly differentiated myofibrils under the regulation of actin-binding proteins (ABPs), which provides a structural basis for the contractile function and morphological change in cardiomyocytes. Previous studies have shown that the functional abnormality of ABPs can contribute to cardiac hypertrophy. Here, we review the function of various actin-binding proteins associated with the development of cardiac hypertrophy, which provides more references for the prevention and treatment of cardiomyopathy.
Keywords: F-actin; actin-binding proteins; cardiac hypertrophy; fetal genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CDC-like kinase 4 deficiency contributes to pathological cardiac hypertrophy by modulating NEXN phosphorylation.Nat Commun. 2022 Jul 30;13(1):4433. doi: 10.1038/s41467-022-31996-9. Nat Commun. 2022. PMID: 35907876 Free PMC article.
-
The actin-organizing formin protein Fhod3 is required for postnatal development and functional maintenance of the adult heart in mice.J Biol Chem. 2018 Jan 5;293(1):148-162. doi: 10.1074/jbc.M117.813931. Epub 2017 Nov 20. J Biol Chem. 2018. PMID: 29158260 Free PMC article.
-
Prdm16 Deficiency Leads to Age-Dependent Cardiac Hypertrophy, Adverse Remodeling, Mitochondrial Dysfunction, and Heart Failure.Cell Rep. 2020 Oct 20;33(3):108288. doi: 10.1016/j.celrep.2020.108288. Cell Rep. 2020. PMID: 33086060
-
Cardiac maturation.J Mol Cell Cardiol. 2024 Feb;187:38-50. doi: 10.1016/j.yjmcc.2023.12.008. Epub 2023 Dec 30. J Mol Cell Cardiol. 2024. PMID: 38160640 Free PMC article. Review.
-
MEF2 in cardiac hypertrophy in response to hypertension.Trends Cardiovasc Med. 2023 May;33(4):204-212. doi: 10.1016/j.tcm.2022.01.002. Epub 2022 Jan 11. Trends Cardiovasc Med. 2023. PMID: 35026393 Review.
Cited by
-
Chronic lactate exposure promotes cardiomyocyte cytoskeleton remodelling.Heliyon. 2024 Jan 16;10(2):e24719. doi: 10.1016/j.heliyon.2024.e24719. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312589 Free PMC article.
-
RICH1 is a novel key suppressor of isoproterenol‑ or angiotensin II‑induced cardiomyocyte hypertrophy.Mol Med Rep. 2024 May;29(5):69. doi: 10.3892/mmr.2024.13193. Epub 2024 Mar 8. Mol Med Rep. 2024. PMID: 38456539 Free PMC article.
-
Ferritinophagy in cardiovascular diseases: mechanisms and potential therapy.Mol Cell Biochem. 2025 Jun 20. doi: 10.1007/s11010-025-05301-3. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40540145 Review.
-
LIM kinases in cardiovascular health and disease.Front Physiol. 2024 Dec 18;15:1506356. doi: 10.3389/fphys.2024.1506356. eCollection 2024. Front Physiol. 2024. PMID: 39744707 Free PMC article. Review.
References
-
- Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., Deswal A., Drazner M.H., Dunlay S.M., Evers L.R., et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

