Integrin Conformational Dynamics and Mechanotransduction
- PMID: 36429013
- PMCID: PMC9688440
- DOI: 10.3390/cells11223584
Integrin Conformational Dynamics and Mechanotransduction
Abstract
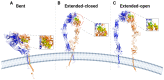
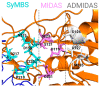
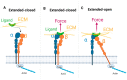
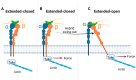
The function of the integrin family of receptors as central mediators of cell-extracellular matrix (ECM) and cell-cell adhesion requires a remarkable convergence of interactions and influences. Integrins must be anchored to the cytoskeleton and bound to extracellular ligands in order to provide firm adhesion, with force transmission across this linkage conferring tissue integrity. Integrin affinity to ligands is highly regulated by cell signaling pathways, altering affinity constants by 1000-fold or more, via a series of long-range conformational transitions. In this review, we first summarize basic, well-known features of integrin conformational states and then focus on new information concerning the impact of mechanical forces on these states and interstate transitions. We also discuss how these effects may impact mechansensitive cell functions and identify unanswered questions for future studies.
Keywords: conformational activation; integrin; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Integrin signaling and mechanotransduction in regulation of somatic stem cells.Exp Cell Res. 2019 May 15;378(2):217-225. doi: 10.1016/j.yexcr.2019.01.027. Epub 2019 Feb 25. Exp Cell Res. 2019. PMID: 30817927 Review.
-
Integrin-mediated mechanotransduction.J Cell Biol. 2016 Nov 21;215(4):445-456. doi: 10.1083/jcb.201609037. Epub 2016 Nov 8. J Cell Biol. 2016. PMID: 27872252 Free PMC article. Review.
-
The mechanical integrin cycle.J Cell Sci. 2009 Jan 15;122(Pt 2):179-86. doi: 10.1242/jcs.042127. J Cell Sci. 2009. PMID: 19118210 Free PMC article. Review.
-
Dynamics and Physics of Integrin Activation in Tumor Cells by Nano-Sized Extracellular Ligands and Electromagnetic Fields.Methods Mol Biol. 2021;2217:197-233. doi: 10.1007/978-1-0716-0962-0_12. Methods Mol Biol. 2021. PMID: 33215383
-
Cell adhesion receptors in mechanotransduction.Curr Opin Cell Biol. 2008 Oct;20(5):551-6. doi: 10.1016/j.ceb.2008.05.005. Epub 2008 Jun 24. Curr Opin Cell Biol. 2008. PMID: 18583124 Free PMC article. Review.
Cited by
-
Application of Funnel Metadynamics to the Platelet Integrin αIIbβ3 in Complex with an RGD Peptide.Int J Mol Sci. 2024 Jun 14;25(12):6580. doi: 10.3390/ijms25126580. Int J Mol Sci. 2024. PMID: 38928286 Free PMC article.
-
Conjecturing about Small-Molecule Agonists and Antagonists of α4β1 Integrin: From Mechanistic Insight to Potential Therapeutic Applications.Biomedicines. 2024 Jan 30;12(2):316. doi: 10.3390/biomedicines12020316. Biomedicines. 2024. PMID: 38397918 Free PMC article. Review.
-
Deciphering the interplay: circulating cell-free DNA, signaling pathways, and disease progression in idiopathic pulmonary fibrosis.3 Biotech. 2025 Apr;15(4):102. doi: 10.1007/s13205-025-04272-y. Epub 2025 Mar 29. 3 Biotech. 2025. PMID: 40165930 Review.
-
Role of the integrin‑β1/TGF‑β1 signaling pathway in the pathogenesis of pelvic organ prolapse: A study on vaginal wall tissue alterations and molecular dysfunction.Mol Med Rep. 2025 Apr;31(4):104. doi: 10.3892/mmr.2025.13469. Epub 2025 Feb 21. Mol Med Rep. 2025. PMID: 39981910 Free PMC article.
-
Mesodermal Derivatives of Pluripotent Stem Cells Route to Scarless Healing.Int J Mol Sci. 2023 Jul 26;24(15):11945. doi: 10.3390/ijms241511945. Int J Mol Sci. 2023. PMID: 37569321 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

