CRISPR-Cas9 Technology for the Creation of Biological Avatars Capable of Modeling and Treating Pathologies: From Discovery to the Latest Improvements
- PMID: 36429042
- PMCID: PMC9688409
- DOI: 10.3390/cells11223615
CRISPR-Cas9 Technology for the Creation of Biological Avatars Capable of Modeling and Treating Pathologies: From Discovery to the Latest Improvements
Abstract
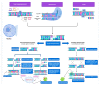
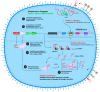
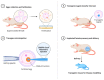
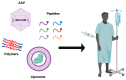
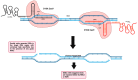
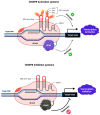
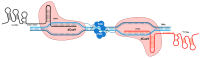
This is a spectacular moment for genetics to evolve in genome editing, which encompasses the precise alteration of the cellular DNA sequences within various species. One of the most fascinating genome-editing technologies currently available is Clustered Regularly Interspaced Palindromic Repeats (CRISPR) and its associated protein 9 (CRISPR-Cas9), which have integrated deeply into the research field within a short period due to its effectiveness. It became a standard tool utilized in a broad spectrum of biological and therapeutic applications. Furthermore, reliable disease models are required to improve the quality of healthcare. CRISPR-Cas9 has the potential to diversify our knowledge in genetics by generating cellular models, which can mimic various human diseases to better understand the disease consequences and develop new treatments. Precision in genome editing offered by CRISPR-Cas9 is now paving the way for gene therapy to expand in clinical trials to treat several genetic diseases in a wide range of species. This review article will discuss genome-editing tools: CRISPR-Cas9, Zinc Finger Nucleases (ZFNs), and Transcription Activator-Like Effector Nucleases (TALENs). It will also encompass the importance of CRISPR-Cas9 technology in generating cellular disease models for novel therapeutics, its applications in gene therapy, and challenges with novel strategies to enhance its specificity.
Keywords: CRISPR-Cas9; disease modeling; gene therapy; genome editing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
CRISPR/Cas9: an advanced tool for editing plant genomes.Transgenic Res. 2016 Oct;25(5):561-73. doi: 10.1007/s11248-016-9953-5. Epub 2016 Mar 24. Transgenic Res. 2016. PMID: 27012546 Review.
-
Genome editing: the road of CRISPR/Cas9 from bench to clinic.Exp Mol Med. 2016 Oct 14;48(10):e265. doi: 10.1038/emm.2016.111. Exp Mol Med. 2016. PMID: 27741224 Free PMC article. Review.
-
[CRISPR/Cas9 technology in disease research and therapy: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Apr 25;37(4):1205-1228. doi: 10.13345/j.cjb.200401. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 33973436 Review. Chinese.
-
Basics of genome editing technology and its application in livestock species.Reprod Domest Anim. 2017 Aug;52 Suppl 3:4-13. doi: 10.1111/rda.13012. Reprod Domest Anim. 2017. PMID: 28815851 Review.
-
CRISPR/Cas9 Platforms for Genome Editing in Plants: Developments and Applications.Mol Plant. 2016 Jul 6;9(7):961-74. doi: 10.1016/j.molp.2016.04.009. Epub 2016 Apr 20. Mol Plant. 2016. PMID: 27108381 Review.
Cited by
-
CRISPR-Cas9 in Cardiovascular Medicine: Unlocking New Potential for Treatment.Cells. 2025 Jan 17;14(2):131. doi: 10.3390/cells14020131. Cells. 2025. PMID: 39851560 Free PMC article. Review.
-
Generation and characterization of CRISPR-Cas9-mediated XPC gene knockout in human skin cells.Sci Rep. 2024 Dec 28;14(1):30879. doi: 10.1038/s41598-024-81675-6. Sci Rep. 2024. PMID: 39730601 Free PMC article.
-
CRISPR/Cas9-Based Modeling of JAK2 V617F Mutation in K562 Cells Reveals Enhanced Proliferation and Sensitivity to Therapeutic Agents.Int J Mol Sci. 2025 May 11;26(10):4600. doi: 10.3390/ijms26104600. Int J Mol Sci. 2025. PMID: 40429745 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

