Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress
- PMID: 36429077
- PMCID: PMC9688738
- DOI: 10.3390/cells11223651
Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress
Abstract
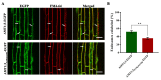
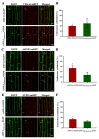
Plants absorb nitrogen from the soil using ammonium transporters (AMTs). Plants can precisely regulate AMT1;3 levels using sophisticated regulatory systems, ensuring adequate nitrogen uptake without hazardous ammonium production. Here, we demonstrated that ubiquitylation can contribute to AMT1;3 degradation under high ammonium stress. Using the ubiquitin site mutant AMT1;3K75R,K233R-EGFP, we demonstrated that the loss of ubiquitination affects the dynamic characteristics of AMT1;3 proteins on the plasma membrane and markedly inhibits the endocytosis of AMT1;3 proteins under high ammonium stress. AMT1;3K75R,K233R-EGFP plants also showed inhibition of protein degradation that targets the vesicular pathway after being exposed to high levels of ammonium. Our findings showed that the dynamic properties, endocytosis, and vesicle trafficking pathways of AMT1;3 proteins are altered in AMT1;3K75R,K233R-EGFP under high ammonium conditions.
Keywords: 3; AMT1; dynamics; endocytosis; ubiquitylation; vesicle trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Miflin B.J., Lea P.J. The pathway of nitrogen assimilation in plants. Phytochemistry. 1976;15:873–885. doi: 10.1016/S0031-9422(00)84362-9. - DOI
-
- Näsholm T., Ekblad A., Nordin A., Giesler R., Högberg M., Högberg P. Boreal forest plants take up organic nitrogen. Nature. 1998;392:914–916. doi: 10.1038/31921. - DOI
-
- Martin M.H., Marschner H. Mineral nutrition of higher plants. J. Ecol. 1988;76:1250. doi: 10.2307/2260650. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

