Effects of Nitisinone on Oxidative and Inflammatory Markers in Alkaptonuria: Results from SONIA1 and SONIA2 Studies
- PMID: 36429096
- PMCID: PMC9688277
- DOI: 10.3390/cells11223668
Effects of Nitisinone on Oxidative and Inflammatory Markers in Alkaptonuria: Results from SONIA1 and SONIA2 Studies
Abstract
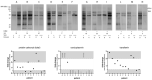
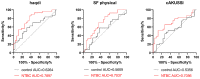
Nitisinone (NTBC) was recently approved to treat alkaptonuria (AKU), but there is no information on its impact on oxidative stress and inflammation, which are observed in AKU. Therefore, serum samples collected during the clinical studies SONIA1 (40 AKU patients) and SONIA2 (138 AKU patients) were tested for Serum Amyloid A (SAA), CRP and IL-8 by ELISA; Advanced Oxidation Protein Products (AOPP) by spectrophotometry; and protein carbonyls by Western blot. Our results show that NTBC had no significant effects on the tested markers except for a slight but statistically significant effect for NTBC, but not for the combination of time and NTBC, on SAA levels in SONIA2 patients. Notably, the majority of SONIA2 patients presented with SAA > 10 mg/L, and 30 patients in the control group (43.5%) and 40 patients (58.0%) in the NTBC-treated group showed persistently elevated SAA > 10 mg/L at each visit during SONIA2. Higher serum SAA correlated with lower quality of life and higher morbidity. Despite no quantitative differences in AOPP, the preliminary analysis of protein carbonyls highlighted patterns that deserve further investigation. Overall, our results suggest that NTBC cannot control the sub-clinical inflammation due to increased SAA observed in AKU, which is also a risk factor for developing secondary amyloidosis.
Keywords: amyloidosis; biomarkers; disease severity; inborn errors of metabolism; inflammation; oxidative stress; protein carbonyls; rare disease; serum amyloid A (SAA).
Conflict of interest statement
The authors declare that they have no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures


References
-
- Ranganath L.R., Milan A.M., Hughes A.T., Dutton J.J., Fitzgerald R., Briggs M.C., Bygott H., Psarelli E.E., Cox T.F., Gallagher J.A., et al. Suitability of nitisinone In alkaptonuria 1 (SONIA 1): An international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid. Ann. Rheum. Dis. 2016;75:362–367. doi: 10.1136/annrheumdis-2014-206033. - DOI - PubMed
-
- Ranganath L.R., Psarelli E.E., Arnoux J.B., Braconi D., Briggs M., Bröijersén A., Loftus N., Bygott H., Cox T.F., Davison A.S., et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): An international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:762–772. doi: 10.1016/S2213-8587(20)30228-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

