In Vitro Antioxidant Activity of Areca Nut Polyphenol Extracts on RAW264.7 Cells
- PMID: 36429198
- PMCID: PMC9689504
- DOI: 10.3390/foods11223607
In Vitro Antioxidant Activity of Areca Nut Polyphenol Extracts on RAW264.7 Cells
Abstract
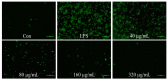
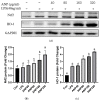
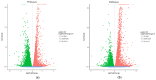
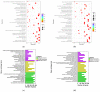
Chewing areca nuts is a popular hobby in the Asian region, and areca nuts are rich in polyphenols, although some alkaloids are included. In this study, we explored the antioxidant activity of areca nut polyphenols (ANP) in lipopolysaccharides (LPS)-stimulated RAW264.7 cells. The results revealed that ANP reduced the level of reactive oxygen species (ROS) in LPS-stimulated RAW264.7 cells and enhanced the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1). RNA-seq analysis showed that ANP down-regulated the transcription of genes related to the cancer pathway at 160 μg/mL, and the inflammatory pathway as well as viral infection pathway at 320 μg/mL. The cellular signaling analysis further revealed that the expressions of these genes were regulated by the mitogen-activated protein kinase (MAPK) pathway, and ANP downregulated the activation of the MAPK signaling pathway stimulated by LPS. Collectively, our findings showed that ANP inhibited the MAPK pathway and activated the Nrf2/HO-1 antioxidant pathways to reduce ROS generation induced by LPS.
Keywords: HO-1; Nrf2; RNA-seq; ROS; areca nut polyphenol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Heatubun C.D., Dransfield J., Flynn T., Tjitrosoedirdjo S.S., Mogea J.P., Baker W.J. A monograph of the betel nut palms (Areca: Arecaceae) of East Malesia. Bot. J. Linn. Soc. 2012;168:147–173. doi: 10.1111/j.1095-8339.2011.01199.x. - DOI
-
- Boniface P., Verma S.K., Cheema H.S., Darokar M.P., Pal A. Evaluation of antimalarial and antimicrobial activites of extract and fractions from Areca catechu. Int. J. Infect. Dis. 2014;21:228–229. doi: 10.1016/j.ijid.2014.03.897. - DOI
-
- Chang Y.-J., Muthukumaran R.B., Chen J.-L., Chang H.-Y., Hung Y.-C., Hu C.-W., Chao M.-R. Simultaneous determination of areca nut- and tobacco-specific alkaloids in saliva by LC-MS/MS: Distribution and transformation of alkaloids in oral cavity. J. Hazard. Mater. 2022;426:128116. doi: 10.1016/j.jhazmat.2021.128116. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

