Eight Months of Serological Follow-Up of Anti-SARS-CoV-2 Antibodies in France: A Study among an Adult Population
- PMID: 36429974
- PMCID: PMC9691066
- DOI: 10.3390/ijerph192215257
Eight Months of Serological Follow-Up of Anti-SARS-CoV-2 Antibodies in France: A Study among an Adult Population
Abstract
Background: Uncertainties remain regarding the nature and durability of the humoral immune response to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Aim: This study investigated immunoglobulin G response and neutralizing activity to evaluate the mean antibody concentrations and response duration induced by each vaccination regimen in a French adult population.
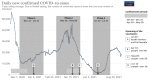
Methods: A study including blood sampling and questionnaires was carried out from November 2020 to July 2021 with three separate follow-up phases. Spike proteins and neutralizing antibodies were quantified using ELISA and a virus-neutralization test.
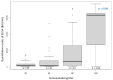
Results: Overall, 295 participants were included. Seroprevalences were 11.5% (n = 34), 10.5% (n = 31), and 68.1% (n = 201) in phases 1, 2, and 3, respectively. Importantly, 5.8% (n = 17) of participants lost their natural antibodies. Antibody response of participants with only a prior infection was 88.2 BAU/mL, significantly lower than those vaccinated, which was 1909.3 BAU/mL (p = 0.04). Moreover, the antibody response of vaccinated participants with a prior infection was higher (3593.8 BAU/mL) than those vaccinated without prior infection (3402.9 BAU/mL) (p = 0.78). Vaccinated participants with or without prior infection had a higher seroneutralization rate (91.0%) than those unvaccinated with prior infection (65.0%).
Conclusion: These results demonstrated that single infection does not confer effective protection against SARS-CoV-2.
Keywords: COVID-19; antibodies; long-lasting immunity; seroconversion; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ministère des Solidarités et de la Santé Le Calendrier de la Campagne Vaccinale Contre la COVID-19. [(accessed on 25 October 2022)]. Mise à Jour le 26 Novembre 2021. Available online: https://solidarites-sante.gouv.fr/archives/archives-presse/archives-comm....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

