Triplin: Functional Probing of Its Structure and the Dynamics of the Voltage-Gating Process
- PMID: 36430243
- PMCID: PMC9693421
- DOI: 10.3390/ijms232213765
Triplin: Functional Probing of Its Structure and the Dynamics of the Voltage-Gating Process
Abstract
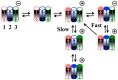
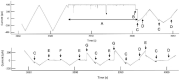
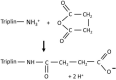
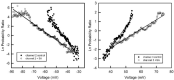
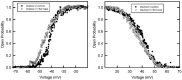
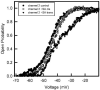
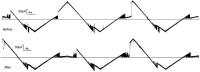
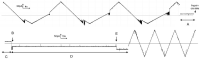
Gram-negative bacteria have a large variety of channel-forming proteins in their outer membrane, generally referred to as porins. Some display weak voltage dependence. A similar trimeric channel former, named Triplin, displays very steep voltage dependence, rivaling that responsible for the electrical excitability of mammals, and high inter-subunit cooperativity. We report detailed insights into the molecular basis for these very unusual properties explored at the single-molecule level. By using chemical modification to reduce the charge on the voltage sensors, they were shown to be positively charged structures. Trypsin cleavage of the sensor eliminates voltage gating by cleaving the sensor. From asymmetrical addition of these reagents, the positively charged voltage sensors translocate across the membrane and are, thus, responsible energetically for the steep voltage dependence. A mechanism underlying the cooperativity was also identified. Theoretical calculations indicate that the charge on the voltage sensor can explain the rectification of the current flowing through the open pores if it is located near the pore mouth in the open state. All results support the hypothesis that one of the three subunits is oriented in a direction opposite to that of the other two. These properties make Triplin perhaps the most complex pore-forming molecular machine described to date.
Keywords: cooperativity; pore; porin; prokaryote; rectification; single channel; trypsin; voltage dependence; voltage sensor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

