Electrochemical Biosensor Designed to Distinguish Tetracyclines Derivatives by ssDNA Aptamer Labelled with Ferrocene
- PMID: 36430261
- PMCID: PMC9698302
- DOI: 10.3390/ijms232213785
Electrochemical Biosensor Designed to Distinguish Tetracyclines Derivatives by ssDNA Aptamer Labelled with Ferrocene
Abstract
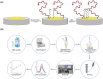
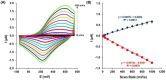
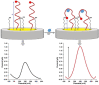
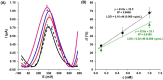
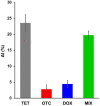
Controlling food safety and preventing the growing spread of antibiotics into food products have been challenging problems for the protection of human health. Hence, the development of easy-to-use, fast, and sensitive analytical methods for the detection of antibiotics in food products has become one of the priorities in the food industry. In this paper, an electrochemical platform based on the ssDNA aptamer for the selective detection of tetracycline has been proposed. The aptasensor is based on a thiolated aptamer, labelled with ferrocene, which has been covalently co-immobilized onto a gold electrode surface with 6-mercaptohexan-1-ol. The changes in the redox activity of ferrocene observed on the aptamer-antibiotics interactions have been the basis of analytical signal generation registered by square-wave voltammetry. Furthermore, the detection of tetracycline-spiked cow milk samples has been successfully demonstrated. The limits of detection (LODs) have been obtained of 0.16 nM and 0.20 nM in the buffer and spiked cow milk, respectively, which exceed the maximum residue level (225 nM) more than 1000 times. The proposed aptasensor offers high selectivity for tetracycline against other structurally related tetracycline derivatives. The developed biosensor characterized by simplicity, a low detection limit, and high reliability shows practical potential for the detection of tetracycline in animal-origin milk.
Keywords: antibiotics; aptamer; cow milk samples; electrochemical biosensor; ferrocene; tetracycline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

