Inhibition of Calpain Attenuates Degeneration of Substantia Nigra Neurons in the Rotenone Rat Model of Parkinson's Disease
- PMID: 36430329
- PMCID: PMC9694996
- DOI: 10.3390/ijms232213849
Inhibition of Calpain Attenuates Degeneration of Substantia Nigra Neurons in the Rotenone Rat Model of Parkinson's Disease
Abstract
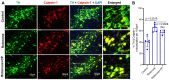
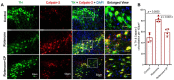
In the central nervous system (CNS), calcium homeostasis is a critical determinant of neuronal survival. Calpain, a calcium-dependent neutral protease, is widely expressed in the brain, including substantia nigra (SN) dopaminergic (DA) neurons. Though calpain is implicated in human Parkinson's disease (PD) and corresponding animal models, the roles of specific ubiquitous calpain isoforms in PD, calpain-1 and calpain-2, remain poorly understood. In this study, we found that both isoforms are activated in a nigrostriatal pathway with increased phosphorylated synuclein following the administration of rotenone in Lewis rats, but calpain isoforms played different roles in neuronal survival. Although increased expression of calpain-1 and calpain-2 were detected in the SN of rotenone-administered rats, calpain-1 expression was not altered significantly after treatment with calpain inhibitor (calpeptin); this correlated with neuronal survival. By contrast, increased calpain-2 expression in the SN of rotenone rats correlated with neuronal death, and calpeptin treatment significantly attenuated calpain-2 and neuronal death. Calpain inhibition by calpeptin prevented glial (astroglia/microglia) activation in rotenone-treated rats in vivo, promoted M2-type microglia, and protected neurons. These data suggest that enhanced expression of calpain-1 and calpain-2 in PD models differentially affects glial activation and neuronal survival; thus, the attenuation of calpain-2 may be important in reducing SN neuronal loss in PD.
Keywords: Parkinson’s disease; alpha-synuclein; calpain; dopaminergic neuron; microglia; rotenone; substantia nigra.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

