MET Signaling Pathways, Resistance Mechanisms, and Opportunities for Target Therapies
- PMID: 36430388
- PMCID: PMC9697723
- DOI: 10.3390/ijms232213898
MET Signaling Pathways, Resistance Mechanisms, and Opportunities for Target Therapies
Abstract
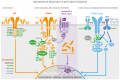
The MET gene, known as MET proto-oncogene receptor tyrosine kinase, was first identified to induce tumor cell migration, invasion, and proliferation/survival through canonical RAS-CDC42-PAK-Rho kinase, RAS-MAPK, PI3K-AKT-mTOR, and β-catenin signaling pathways, and its driver mutations, such as MET gene amplification (METamp) and the exon 14 skipping alterations (METex14), activate cell transformation, cancer progression, and worse patient prognosis, principally in lung cancer through the overactivation of their own oncogenic and MET parallel signaling pathways. Because of this, MET driver alterations have become of interest in lung adenocarcinomas since the FDA approval of target therapies for METamp and METex14 in 2020. However, after using MET target therapies, tumor cells develop adaptative changes, favoring tumor resistance to drugs, the main current challenge to precision medicine. Here, we review a link between the resistance mechanism and MET signaling pathways, which is not only limited to MET. The resistance impacts MET parallel tyrosine kinase receptors and signals shared hubs. Therefore, this information could be relevant in the patient's mutational profile evaluation before the first target therapy prescription and follow-up to reduce the risk of drug resistance. However, to develop a resistance mechanism to a MET inhibitor, patients must have access to the drugs. For instance, none of the FDA approved MET inhibitors are registered as such in Chile and other developing countries. Constant cross-feeding between basic and clinical research will thus be required to meet future challenges imposed by the acquired resistance to targeted therapies.
Keywords: NSCLC; actionable mutations; driver mutations; precision medicine; resistance mutations; target therapies.
Conflict of interest statement
R.A. received honoraria for conferences, advisory boards, and educational activities from Roche and Janssen and grants and support for scientific research from Pfizer, Roche & Thermo Fischer Scientific. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Mi J., Hooker E., Balog S., Zeng H., Johnson D.T., He Y., Yu E.J., Wu H., Le V., Lee D.H., et al. Activation of hepatocyte growth factor/MET signaling initiates oncogenic transformation and enhances tumor aggressiveness in the murine prostate. J. Biol. Chem. 2018;293:20123–20136. doi: 10.1074/jbc.RA118.005395. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

