Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers
- PMID: 36430393
- PMCID: PMC9692705
- DOI: 10.3390/ijms232213914
Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers
Abstract
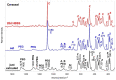
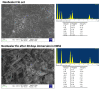
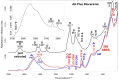
The aim of the study was to analyze the chemical−physical properties and bioactivity (apatite-forming ability) of three recently introduced premixed bioceramic root canal sealers containing varied amounts of different calcium silicates (CaSi): a dicalcium and tricalcium silicate (1−10% and 20−30%)-containing sealer with zirconium dioxide and tricalcium aluminate (CERASEAL); a tricalcium silicate (5−15%)-containing sealer with zirconium dioxide, dimethyl sulfoxide and lithium carbonate (AH PLUS BIOCERAMIC) and a dicalcium and tricalcium silicate (10% and 25%)-containing sealer with calcium aluminate, tricalcium aluminate and tantalite (NEOSEALER FLO). An epoxy resin-based sealer (AH PLUS) was used as control. The initial and final setting times, radiopacity, flowability, film thickness, open pore volume, water absorption, solubility, calcium release and alkalizing activity were tested. The nucleation of calcium phosphates and/or apatite after 28 days aging in Hanks balanced salt solution (HBSS) was evaluated by ESEM-EDX, vibrational IR and micro-Raman spectroscopy. The analyses showed for NeoSealer Flo and AH Plus the longest final setting times (1344 ± 60 and 1300 ± 60 min, respectively), while shorter times for AH Plus Bioceramic and Ceraseal (660 ± 60 and 720 ± 60 min, respectively). Radiopacity, flowability and film thickness complied with ISO 6876/12 for all tested materials. A significantly higher open pore volume was observed for NeoSealer Flo, AH Plus Bioceramic and Ceraseal when compared to AH Plus (p < 0.05), significantly higher values were observed for NeoSealer Flo and AH Plus Bioceramic (p < 0.05). Ceraseal and AH Plus revealed the lowest solubility. All CaSi-containing sealers released calcium and alkalized the soaking water. After 28 days immersion in HBSS, ESEM-EDX analyses revealed the formation of a mineral layer that covered the surface of all bioceramic sealers, with a lower detection of radiopacifiers (Zirconium for Ceraseal and AH Plus Bioceramic, Tantalum for NeoSealer Flo) and an increase in calcium, phosphorous and carbon. The calcium phosphate (CaP) layer was more evident on NeoSealer Flo and AH Plus Bioceramic. IR and micro-Raman revealed the formation of calcium carbonate on the surface of all set materials. A thin layer of a CaP phase was detected only on AH Plus Bioceramic and NeoSealer Flo. Ceraseal did not show CaP deposit despite its highest calcium release among all the tested CaSi-containing sealers. In conclusion, CaSi-containing sealers met the required chemical and physical standards and released biologically relevant ions. Slight/limited apatite nucleation was observed in relation to the high carbonation processes.
Keywords: apatite nucleation; bioactivity; bioceramics; calcium phosphate nucleation; calcium silicates; calcium silicates cements; endodontic sealers; root canal sealers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Primus C., Gutmann J.L., Tay F.R., Fuks A.B. Calcium silicate and calcium aluminate cements for dentistry reviewed. J. Am. Ceram. Soc. 2022;105:1841–1863. doi: 10.1111/jace.18051. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

