The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia
- PMID: 36430426
- PMCID: PMC9694174
- DOI: 10.3390/ijms232213947
The Impact of Duodenal Mucosal Vulnerability in the Development of Epigastric Pain Syndrome in Functional Dyspepsia
Abstract
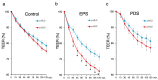
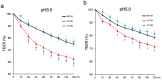
An unidentified cause of functional dyspepsia (FD) is closely associated with medication resistance. Acid suppression is a traditional and preferential method for the treatment of FD, but the efficacy of this treatment varies between epigastric pain syndrome (EPS) and postprandial syndrome (PDS): it is efficient in the former but not much in the latter. Transepithelial electrical resistance (TEER), a surrogate of mucosal barrier function, was measured under pH 3 and pH 5 acidic conditions using duodenal biopsy specimens obtained from the patients with EPS and PDS and asymptomatic healthy controls. The infiltration of inflammatory cells to the duodenal mucosa was accessed by immunohistochemical analysis. The duodenal mucosal TEER in EPS patients was decreased by exposure to the acidic solution compared to that of the controls and the PDS patients. The decrease in TEER of the EPS patients was observed even under pH 5 weak acidic condition and was correlated to degree of the epigastric pain. Moreover, the duodenal mucosa of EPS patients presented an increase in mast cells and plasma cells that expressed Ig-E. Duodenal mucosal vulnerability to acid is likely to develop EPS.
Keywords: acid; duodenal mucosa; epigastric pain syndrome; mucosal vulnerability; transepithelial electrical resistance.
Conflict of interest statement
There was no disclosure of financial arrangements related to the research or assistance with manuscript preparation.
Figures

References
-
- van Oudenhove L., Holvoet L., Vandenberghe J., Vos R., Tack J. Do we have an alternative for the Rome III gastroduodenal symptom-based subgroups in functional gastroduodenal disorders? A cluster analysis approach. Neurogastroenterol. Motil. 2011;23:730–738. doi: 10.1111/j.1365-2982.2011.01703.x. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

