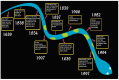
Amyloidogenesis: What Do We Know So Far?
- PMID: 36430450
- PMCID: PMC9695042
- DOI: 10.3390/ijms232213970
Amyloidogenesis: What Do We Know So Far?
Abstract
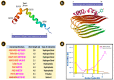
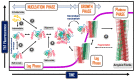
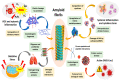
The study of protein aggregation, and amyloidosis in particular, has gained considerable interest in recent times. Several neurodegenerative diseases, such as Alzheimer's (AD) and Parkinson's (PD) show a characteristic buildup of proteinaceous aggregates in several organs, especially the brain. Despite the enormous upsurge in research articles in this arena, it would not be incorrect to say that we still lack a crystal-clear idea surrounding these notorious aggregates. In this review, we attempt to present a holistic picture on protein aggregation and amyloids in particular. Using a chronological order of discoveries, we present the case of amyloids right from the onset of their discovery, various biophysical techniques, including analysis of the structure, the mechanisms and kinetics of the formation of amyloids. We have discussed important questions on whether aggregation and amyloidosis are restricted to a subset of specific proteins or more broadly influenced by the biophysiochemical and cellular environment. The therapeutic strategies and the significant failure rate of drugs in clinical trials pertaining to these neurodegenerative diseases have been also discussed at length. At a time when the COVID-19 pandemic has hit the globe hard, the review also discusses the plausibility of the far-reaching consequences posed by the virus, such as triggering early onset of amyloidosis. Finally, the application(s) of amyloids as useful biomaterials has also been discussed briefly in this review.
Keywords: Aβ peptide; COVID-19 and amyloidosis; amyloid precursor protein; amyloid related diseases; amyloid structure analysis; amyloids; fibril formation; physical techniques in amyloid analysis; protein aggregation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

