(-)-Epigallocatechin-3-Gallate Prevents IL-1β-Induced uPAR Expression and Invasiveness via the Suppression of NF-κB and AP-1 in Human Bladder Cancer Cells
- PMID: 36430487
- PMCID: PMC9697952
- DOI: 10.3390/ijms232214008
(-)-Epigallocatechin-3-Gallate Prevents IL-1β-Induced uPAR Expression and Invasiveness via the Suppression of NF-κB and AP-1 in Human Bladder Cancer Cells
Abstract
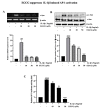
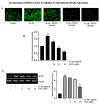
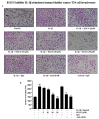
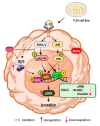
(-)-Epigallocatechin-3-O-gallate (EGCG), a primary green tea polyphenol, has powerful iron scavengers, belongs to the family of flavonoids with antioxidant properties, and can be used to prevent cancer. Urokinase-type plasminogen activator receptors (uPARs) are glycosylphosphatidylinositol (GPI)-anchored cell membrane receptors that have crucial roles in cell invasion and metastasis of several cancers including bladder cancer. The mechanism of action of EGCG on uPAR expression has not been reported clearly yet. In this study, we investigated the effect of EGCG on interleukin (IL)-1β-induced cell invasion and uPAR activity in T24 human bladder cancer cells. Interestingly, nuclear factor (NF)-κB and activator protein (AP)-1 transcription factors were critically required for IL-1β-induced high uPAR expression, and EGCG suppressed the transcriptional activity of both the ERK1/2 and JNK signaling pathways with the AP-1 subunit c-Jun. EGCG blocked the IL-1β-stimulated reactive oxygen species (ROS) production, in turn suppressing NF-κB signaling and anti-invasion effects by inhibiting uPAR expression. These results suggest that EGCG may exert at least part of its anticancer effect by controlling uPAR expression through the suppression of ERK1/2, JNK, AP-1, and NF-κB.
Keywords: AP-1; EGCG; IL-1β; NF-κB; ROS; Urokinase-type plasminogen activator receptor (uPAR); cell invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Khoi P.N., Li S., Thuan U.T., Sah D.K., Kang T.W., Nguyen T.T., Lian S., Xia Y., Jung Y.D. Lysophosphatidic Acid Upregulates Recepteur D’origine Nantais Expression and Cell Invasion via Egr-1, AP-1, and NF-kappaB Signaling in Bladder Carcinoma Cells. Int. J. Mol. Sci. 2020;21:304. doi: 10.3390/ijms21010304. - DOI - PMC - PubMed
-
- DeGeorge K.C., Holt H.R., Hodges S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician. 2017;96:507–514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

