Effect of the First Feeding on Enterocytes of Newborn Rats
- PMID: 36430658
- PMCID: PMC9699143
- DOI: 10.3390/ijms232214179
Effect of the First Feeding on Enterocytes of Newborn Rats
Abstract
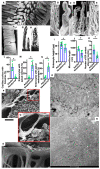
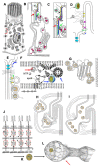
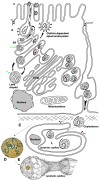
The transcytosis of lipids through enterocytes occurs through the delivery of lipid micelles to the microvilli of enterocytes, consumption of lipid derivates by the apical plasma membrane (PM) and then their delivery to the membrane of the smooth ER attached to the basolateral PM. The SER forms immature chylomicrons (iChMs) in the ER lumen. iChMs are delivered at the Golgi complex (GC) where they are subjected to additional glycosylation resulting in maturation of iChMs. ChMs are secreted into the intercellular space and delivered into the lumen of lymphatic capillaries (LCs). The overloading of enterocytes with lipids induces the formation of lipid droplets inside the lipid bilayer of the ER membranes and transcytosis becomes slower. Here, we examined components of the enterocyte-to-lymphatic barriers in newly born rats before the first feeding and after it. In contrast to adult animals, enterocytes of newborns rats exhibited apical endocytosis and a well-developed subapical endosomal tubular network. These enterocytes uptake membranes from amniotic fluid. Then these membranes are transported across the polarized GC and secreted into the intercellular space. The enterocytes did not contain COPII-coated buds on the granular ER. The endothelium of blood capillaries situated near the enterocytes contained only a few fenestrae. The LCs were similar to those in adult animals. The first feeding induced specific alterations of enterocytes, which were similar to those observed after the lipid overloading of enterocytes in adult rats. Enlarged chylomicrons were stopped at the level of the LAMP2 and Neu1 positive post-Golgi structures, secreted, fused, delivered to the interstitial space, captured by the LCs and transported to the lymph node, inducing the movement of macrophages from lymphatic follicles into its sinuses. The macrophages captured the ChMs, preventing their delivery into the blood.
Keywords: Golgi complex; enterocytes; lipid overloading; lymphatic capillaries; newborn; transcytosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
Structure of the enterocyte transcytosis compartments during lipid absorption.Histochem Cell Biol. 2020 Jun;153(6):413-429. doi: 10.1007/s00418-020-01851-3. Epub 2020 Mar 11. Histochem Cell Biol. 2020. PMID: 32162136
-
Cellular and sub-cellular mechanisms of lipid transport from gut to lymph.Tissue Cell. 2021 Oct;72:101529. doi: 10.1016/j.tice.2021.101529. Epub 2021 Apr 20. Tissue Cell. 2021. PMID: 33915359 Review.
-
Formation and transport of chylomicrons by enterocytes to the lymphatics.Am J Physiol. 1986 Jun;250(6 Pt 1):G715-26. doi: 10.1152/ajpgi.1986.250.6.G715. Am J Physiol. 1986. PMID: 3521320 Review.
-
[The origin and transport of chylomicrons in enterocytes in chickens].Vet Med (Praha). 1982 May;27(5):295-308. Vet Med (Praha). 1982. PMID: 6808756 Czech.
-
Characteristics and functions of lipid droplets and associated proteins in enterocytes.Exp Cell Res. 2016 Jan 15;340(2):172-9. doi: 10.1016/j.yexcr.2015.09.018. Epub 2015 Sep 30. Exp Cell Res. 2016. PMID: 26431584 Review.
Cited by
-
Intracellular Membrane Transport in Vascular Endothelial Cells.Int J Mol Sci. 2023 Mar 17;24(6):5791. doi: 10.3390/ijms24065791. Int J Mol Sci. 2023. PMID: 36982865 Free PMC article. Review.
-
New Interpretations for Sprouting, Intussusception, Ansiform, and Coalescent Types of Angiogenesis.Int J Mol Sci. 2024 Aug 6;25(16):8575. doi: 10.3390/ijms25168575. Int J Mol Sci. 2024. PMID: 39201261 Free PMC article. Review.
-
Role of Endothelial Regeneration and Overloading of Enterocytes with Lipids in Capturing of Lipoproteins by Basement Membrane of Rat Aortic Endothelium.Biomedicines. 2022 Nov 8;10(11):2858. doi: 10.3390/biomedicines10112858. Biomedicines. 2022. PMID: 36359378 Free PMC article.
-
COVID-19 Biogenesis and Intracellular Transport.Int J Mol Sci. 2023 Feb 24;24(5):4523. doi: 10.3390/ijms24054523. Int J Mol Sci. 2023. PMID: 36901955 Free PMC article. Review.
-
Mechanisms of Formation of Antibodies against Blood Group Antigens That Do Not Exist in the Body.Int J Mol Sci. 2023 Oct 10;24(20):15044. doi: 10.3390/ijms242015044. Int J Mol Sci. 2023. PMID: 37894724 Free PMC article.
References
-
- Sesorova I.S., Karelina N.R., Kazakova T.E., Parashuraman S., Zdorikova M.A., Dimov I.D., Seliverstova E.V., Beznoussenko G.V., Mironov A.A. Structure of the enterocyte transcytosis compartments during lipid absorption. Histochem. Cell Biol. 2020;153:413–429. doi: 10.1007/s00418-020-01851-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

